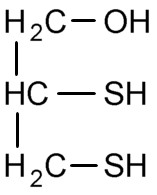
Figure 3. BAL, British anti-Lewisite
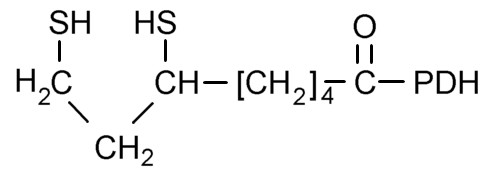
Figure 4. Lipoic acid attached to pyruvate dehydrogenase (PDH)

Figure 3. BAL, British anti-Lewisite |
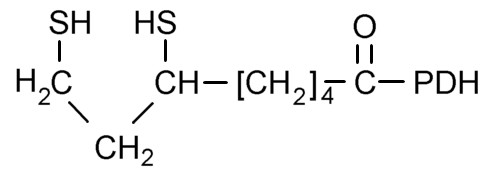
Figure 4. Lipoic acid attached to pyruvate dehydrogenase (PDH) |
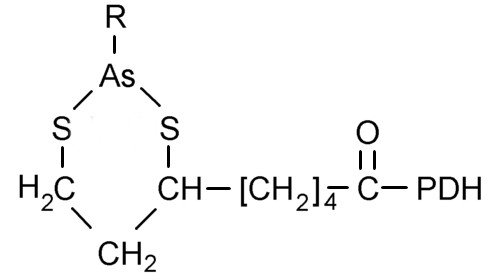
Figure 5. Six-membered ring formed by arsenic and lipoic acid |
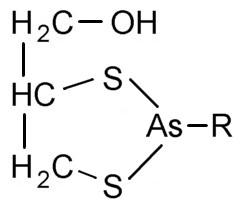
Figure 6. Favored 5-membered ring of BAL and arsenic |