![]()
Colchicine
The poison that's also a treatment for gout
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month April 2015
Also available: HTML version.
![]()
![The Gout by James Gillray Gillray [Public domain], via Wikimedia Commons The Gout](the_gout.jpg)
|
ColchicineThe poison that's also a treatment for gout
Simon Cotton
Molecule of the Month April 2015
| ![The Gout by James Gillray Gillray [Public domain], via Wikimedia Commons The Gout](the_gout.jpg)
|
It’s an image of gout, drawn by the famous English satirical artist, James Gillray (1757-1815).
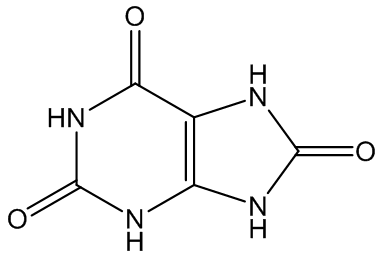
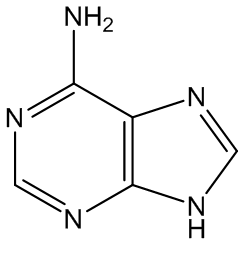
Gout is caused by crystallisation of uric acid in the joints - often for some reason in the big toe - and is a type of inflammatory arthritis. Uric acid is naturally formed in the body by breakdown of purine bases (adenine, guanine) via xanthine. It is mainly removed via the kidneys, but sometimes there is more than a body can cope with, and the excess build up as small, irritating crystals in joints.
 |
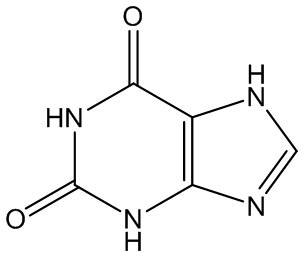 |
Uric acid | Xanthine |
 |
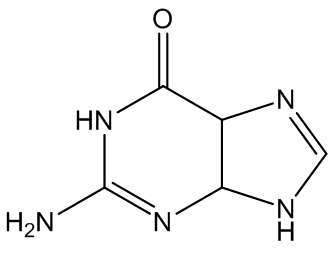 |
Adenine | Guanine |
 And there’s a good treatment?
And there’s a good treatment?Traditionally colchicine is used.
Perhaps the best testimony is due to the Rev. Sydney Smith (1771-1845, image right), the famous writer and wit (who once said "I never read a book before reviewing it; it prejudices a man so.") In a letter dated December 1838 he wrote: "On Sunday I was on crutches utterly unable to put foot on the ground, on Tuesday I walked 4 miles - such is the power of Colchicum." It was Smith who commented that gout was "the only enemy that I do not wish to have at my feet". On another occasion, observing some of his autumn crocuses in flower, he remarked, "There, who would guess the virtue of that little plant? But I find the power of Colchicum so great that if I feel a little gout coming on, I go into the garden and hold out my little toe to that plant, and it gets well immediately".
|
How do you mean?
That's right, and it was years before the structure of colchicine was sorted out. It was first isolated in 1820 by two French chemists Pierre Joseph Pelletier and Jean Bienaimé Caventou. They are better known for their isolation of quinine (as well as strychnine).
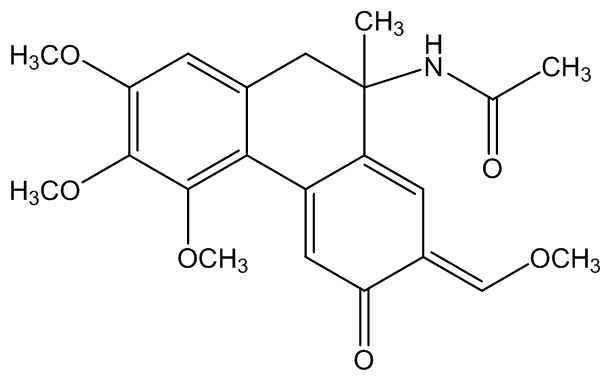
Windaus' proposed, but incorrect, structure of colchicine
Originally a structure based on six-membered rings (above) was suggested by Windaus, and it was not until 1945 that M.J.S. Dewar (correctly) suggested the presence of seven-membered rings, confirmed subsequently by X-ray diffraction.
If you define alkaloids as “nitrogenous organic compounds of plant origin” (made from breakdown of amino acids), the answer is yes. Some people expect all alkaloids to be basic. Colchinine’s nitrogen atom is part of an amide group, which means that it can’t be protonated, so colchicine is not a base, and by this count not an alkaloid.
It comes from the autumn crocus, or meadow saffron (Colchicum autumnale), which in turn originates in the Colchis region (Greek Koλξoζ) on the Black Sea coast of what is now Georgia. A few other plants contain it, such as Gloriosa superba (glory lily).
 |
 |
Meadow saffron (Colchicum autumnale) |
Glory lily (Gloriosa superba) |
Colchis is famous in classical literature and mythology. Colchicum autumnale, the yellow crocus of Colchis, is associated in legend with Medea, the sorceress daughter of Aeëtes, King of Colchis. She used the poison from its roots – which included colchicine – among her potions. The goddess Aphrodite made Medea fall in love with Jason, leader of the Argonauts, who relied on her assistance in his quest for the Golden Fleece in overcoming the tasks set for him by the king. Some have identified Colchicum autumnale with the Golden Fleece itself. Jason and Medea sailed off together into the sunset, but the story does not have a happy ending, as subsequently Jason dumps Medea for a younger woman, Glauce (daughter of the King of Corinth). In revenge, Medea killed Glauce with a poisoned dress and also in some accounts killed her children with colchicine.
 |
 |
Painting of Jason and Medea |
Engaving of Jason on the Douris cup (5th century BC) |
Indeed. Several people have died through mistaking the autumn crocus for wild garlic. According to some accounts, the symptoms are similar to cholera. The lethal dose varies, with some people killed by as little as 7 mg, others surviving doses of 60 mg. It has also been used as a poison and as an instrument of suicide. Arkady Vaksberg has alleged that the influential Russian businessman Roman Tsepov died in 2004 of colchicine poisoning, and Catherine Wilson, the last woman to be hanged publicly in the UK (1862), used colchicine as her preferred weapon of death. She is believed to have carried out at least seven murders. A few other cases are known of homicide by colchicine.

Newspaper article describing the public execution of Catherine Wilson for murder in London in 1862 [Harvard University Library].
Colchicine has been around for some 3000 years – it was described by ancient Greek writers. It is said that it was introduced into the USA by Benjamin Franklin, who had taken it while in Paris. Mind you, it took the American Food and Drug Administration (FDA) until 2009 to "confirm" that it was an effective treatment for gout.
Colchicine binds to β-tubulin, a protein that joins with α-tubulin forming a dimer. When colchicine is bound, the dimer is curved, not linear as usual, and this inhibits the process of assembly of tubulin into microtubules, the key components of the cytoskeleton. In turn, this affects the formation of the spindle in the nucleus of eukaryotic cells, stopping mitosis from occurring and leading to cell death. One consequence of this which has attracted attention is the fact that cancer cells undergo mitosis significantly faster than normal cells so that cancer cells are more liable to be poisoned by colchicine than normal cells. But its high toxicity is one factor mitigating against the potential of colchicine as an anticancer drug.
![]()
Chapman and Hall Combined Chemical Dictionary compound code number: CFR10-M (props, bibliography)
![]()