EDTA - A Molecule with a Complex Story
Scott A. Sinex
Prince George's Community College
Molecule of the Month - March 2004
What do beer, the special sauce on a McDonald's Big Mac, and blue colored shampoos such as Aloe Vera or Revlon Aquamarine have in common?
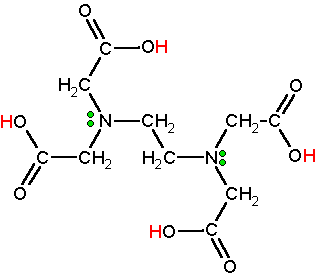
EDTA or ethylenediaminetetraacetic acid is a novel molecule for complexing metal ions. It is a polyprotic acid containing four carboxylic acid groups (acidic hydrogens are red) and two amine groups with lone pair electrons (green dots). The classic structural formula is given below. EDTA is synthesized on an industrial scale from ethylenediamine, formaldehyde, and a source of cyanide (HCN or NaCN). Click here for the industrial reactions to open in a new window. On a worldwide basis over 100,000 metric tons are produced annually.

Besides the four carboxylic group hydrogens, EDTA can add two more hydrogens onto the amine groups. The structures of the fully protonated form (left), the typical form found in many textbook (center, matching the 2D structure above), and the fully deprotonated (all acidic H's removed) form (right) are given below.
|
forms at very low pH or |
classic form |
forms at very high pH or |
The unusual property of EDTA is its ability to chelate or complex metal ions in 1:1 metal-to-EDTA complexes. The fully deprotonated form (all acidic hydrogens removed) of EDTA binds to the metal ion. The equilibrium or formation constants for most metals, especially the transition metals, are very large, hence the reactions are shifted to the complex. Many of the reactions are pH dependent, especially the weaker forming complexes with Ca+2 or Mg+2.
M+n
+ Y-4 ![]() MYn-4 Kf = (MYn-4)/(M+n)(Y-4)
MYn-4 Kf = (MYn-4)/(M+n)(Y-4)
Metal analysis can be done by titration with EDTA and the use of a metal ion indicator. The pioneering work with EDTA was done by Gerold Schwarzenbach in the 1940's. The common reagent for making EDTA solutions is Na2H2Y.2H2O. Click here for a list of formation constant (Kf) values. The values of Kf increase with the charge on the metal ion and as ionic radius decreases with constant charge. Water hardness, mostly from dissolved Ca+2 and Mg+2, is determined by EDTA titration at pH = 10 - click here for a typical experiment.
The structure of a classical complex of Fe+3 with EDTA is shown below. This is EDTA acting as a hexadentate ligand or all six sites on the ETDA bind to the metal ion. How would you describe the geometry of the octahedral Fe+3 in the complex?
The octahedral coordination of the Fe-EDTA (left) and many other such complexes are very strained. The space-fill version of the structure (right) shows how crowded the structure appears. Consider the structure of Cr+3 with EDTA shown below. What is different for the Cr-EDTA complex compared to the Fe-EDTA complex?
A number of metal-EDTA complexes have been reported to have the EDTA acting as a pentadentate ligand (only five sites on EDTA bind, one carboxylic group does NOT). A water molecule or another ligand is in the sixth site, so the complexes are still octahedral in geometry.
So let's examine the formation of the complex. The animation below was produced in Spartan '04 by placing an Fe+3 (green) next to the EDTA with acidic H's removed (fully deprotonated as Y-4) and then minimizing the energy. Note that EDTA forms only five bonds to the Fe+3, as the complex forms (center image is complex at end of animation). The image to the right has a molecule of water added, plus the EDTA has picked up a hydrogen ion on the carboxylic acid group not bound to the iron, and energy minimized to form the six coordinate complex.
|
|
||
| Animation of Y-4
complexing Fe+3 This is an avi file, so you'll need to have Real Player or Windows Media Player configured accordingly in order to see it. |
complex with EDTA as a pentadentate ligand |
octehedral complex with |
Because of its strong complexing ability for most metal ions, it is used in the food industry as a sequestering agent. The complexing of the metal ion may prevent further reactions, such as binding metals that are cofactors for enzymes, or just remove a metallic taste, such as metal contamination added during processing. See the Dow Chemical site on the use of their commercial product - Versene. Some typical examples are given below.
Recent studies have shown that NaFeEDTA and Na2EDTA added to typical iron fortification compounds in cereals increased the absorption of iron in adult humans.
This same property allows EDTA use for incidents of lead poisoning by the medical profession. The formation constant for Pb-EDTA complex is 1018. Intravenous injection of Na2CaEDTA solution is given at 25 mg/kg body mass/day over 6 hours for 5 days when blood lead levels go over 45 mg/dL. The Pb+2 ion replaces the Ca+2 ion in the complex because the formation constant for the lead complex is greater than the calcium complex.
Pb+2
+ CaY-2 ![]() PbY-2 + Ca+2
K ~ 108
PbY-2 + Ca+2
K ~ 108
The five day limit is there to prevent Zn+2 depletion, since the Zn+2 ion replaces the Ca+2 ion in the complex too. EDTA is added to stored blood in blood banks as an anticoagulant to bind Ca+2 ion. Other reported uses of EDTA in medicine do not have a proven clinical basis. Click here for more information.
Another major use of EDTA has been in detergents to act as a builder (chelates metals) especially as a replacement for phosphates, a major nutrient in wastewater. However, a problem with EDTA is its inability to biodegrade in the environment. EDTA is found in many natural waters and occurs at higher levels in wastewater effluents. Western European countries have banned the use of EDTA in detergents. For a complete discussion of the chelating agents in the environment - click here. EDDS (S, S'-ethylenediaminedisuccinic acid), a structural isomer of EDTA, has been used as a biodegradable substitute. EDDS is a good complexing agent and is broken down during wastewater treatment processes. This is a contribution to green chemistry by making a minor structural change. The cumulative production of EDDS by Innospec Inc. (formerly Octel) surpassed 10,000 metric tons in 2002.
|
|
||
| EDDS structure showing chiral carbons in red - both must be S. l-Aspartic acid in green box. | biodegradable S, S'- EDDS molecule | octahedral FeEDDS- complex |
EDTA is added to many commercial beers to stabilize foaming, taking advantage of the surfactant properties of EDTA, and used to remove scale by complexing calcium from calcium carbonate that forms on the processing equipment.
The blue color of the Cu-EDTA complex is used in many shampoos. See the FDA use of disodium EDTA-copper in cosmetics.
Ethylenediaminetetraacetic Acid and Related Chelating Agents in Ullmann's Encyclopedia of Industrial Chemistry Vol. A10
What's that stuff: Food Preservatives, C&EN, 11 November 2002 http://pubs.acs.org/cen/science/8045/8045sci2.html
CSPI's
Guide to Food Additives
http://www.cspinet.org/reports/chemcuisine.htm
The Brewer's Handbook
http://www.beer-brewing.com
Lead Poisoning in The Merck Manual
http://www.merck.com/mmpe/sec21/ch326/ch326j.html
UK Awards for Green Chemical
Technology to Octel Performance Chemicals
http://www.chemsoc.org/pdf/gcn/Octel_Award.pdf
A Brief History of Inorganic
Classical Analysis
http://www.cstl.nist.gov/nist839/839.01/beckhist.pdf
Storing Up Trouble
http://www.chemsoc.org/chembytes/ezine/1998/store.htm
EDTA: The Chelating Agent under Environmental Scrutiny http://www.scielo.br/pdf/qn/v26n6/a20v26n6.pdf
Chemical Speciation of EDDS and its
metal complexes
http://www.scilet.com/Papers/csb/csb113/CBSWhitburn.pdf
An Evaluation of EDTA Compounds for
Iron Fortification of Cereal-based Foods
Br. J. Nutr. 84 (6), 903-910 (2000)
Emerging Chemical Drinking Water Contaminants in Identifying Future Drinking Water Contaminants http://www.nap.edu/books/0309064325/html/112.html
Biogeochemistry of Chelating Agents, edited by B. Nowack and J. VanBriesen, ACS Symposium Series Vol. 910, 2005 http://www.ito.ethz.ch/SoilProt/staff/nowack/book_toc.html
Just for FUN: See the EDTA song - click here.
 Feedback
welcome - click on mail
box.
1 March 2004
Feedback
welcome - click on mail
box.
1 March 2004
revised 1 August 2007
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5437117]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5437117]