THE CHANNELS IN FERRITIN
Note: Please use the scroll bar to see the entire link.
Introduction
The role of the channels in both the iron-formation and
iron-removal processes is dependent on the structural differences
between the 2 channels. In this link, the residues that comprise
the channels and the structural differences between these
residues are examined.
The intersection of the subunits form two (2) types of
channels:
- The 4-fold channel, which is lined with leucine residues,
is hydrophobic;
- The 3-fold channel, which is lined with glutamate and
aspartate residues, is hydrophilic.
4-FOLD CHANNEL
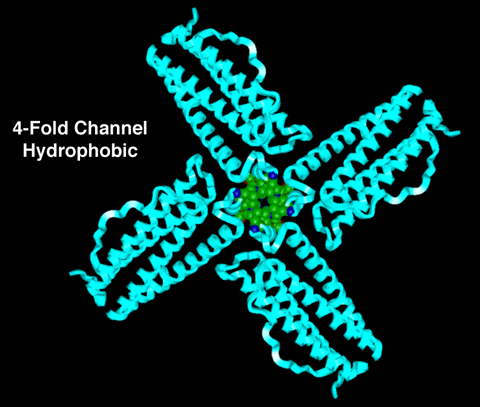 |
Figure 1:
This is a molecular model of the 4-fold channel with
the four (4) intersecting subunits represented as ribbons
and the sidechains lining the channel represented as CPK
models. (In this picture, the ribbons have the same color
code as the ribbons in Figure 1 in the main section of
this tutorial.) The carbon atoms in the sidechains lining
the channel are shown in green and represented as CPK
models. Hydrogens are omitted from the picture for visual
clarity.
Notice that there are no polar groups containing
oxygen lining this channel. This results in the channel
having a hydrophobic nature.
Note: The carbon atoms are green, the nitrogen
atoms are blue, and the oxygen atoms are red in the CPK
model.
|
3-FOLD CHANNEL
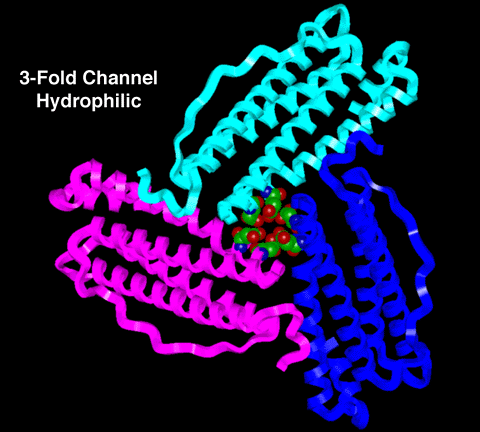 |
Figure 2:
This is a molecular model of the 3-fold channel with
the three (3) intersecting subunits represented as
ribbons. (In this picture, the ribbons have the same
color code as the ribbons in Figure 1 in the main section
of this tutorial.) The amino-acid residues lining the
channel are shown as CPK models.
Notice the polarity of the carboxylate sidechains on
the residues lining the 3-fold channel; the polar nature
of these sidechains results in this channel being
hydrophilic.
Note: The carbon atoms are green, the nitrogen
atoms are blue, and the oxygen atoms are red in the CPK
model.
|
View the three (3) residues involved by clicking here.
Questions
- The molecular models of the 3 residues show that all
three of the residues contain polar groups. This seems to
lead to a contradiction since one channel is hydrophilic
(lined with polar groups) and the other channel is
hydrophobic (lined with nonpolar groups). How can this
occur? (HINT: Look in the peptide
and residue links.)
- How do the structural differences in the 2 channels
affect the roles that the 2 channels have on the
iron-removal mechanism? ( HINT: Look in this link.)
- Why is the NH3+
group (i.e., amine) on the residues not used
to make the 4-fold channel hydrophilic? (HINT: Look in
the peptide link
and this link.)
Return to
Structural-Background Section of Tutorial
Return to
Questions on the Background Section of Tutorial
Return to
Questions on the Iron-removal Section of Tutorial

