|
Fluorine (F2)The most reactive non-metal
Mike Thompson and Hugh Campbell
Also available: HTML version.
|
|
Fluorine (F2)The most reactive non-metal
Mike Thompson and Hugh Campbell
Also available: HTML version.
|
 It was in 1886 that the French scientist Henri Moissan (photo, right) was the first to prepare and isolate pure fluorine. For this and other important work Moissan was awarded the Nobel prize in 1906. In the words of the Nobel committee, "in recognition of the great services rendered by him in his investigation and isolation of the element fluorine, and for the adoption in the service of science of the electric furnace called after him". His original paper entitled Le Fluor et ses Composés is available online. The French scientist, André Ampère coined the name fluorine in 1812. The name originates from the Latin word 'fluo' meaning flow. Other famous scientists such as Humphry Davey had ‘seen’ fluorine but did not manage to successfully isolate it, as it reacted with their apparatus.
It was in 1886 that the French scientist Henri Moissan (photo, right) was the first to prepare and isolate pure fluorine. For this and other important work Moissan was awarded the Nobel prize in 1906. In the words of the Nobel committee, "in recognition of the great services rendered by him in his investigation and isolation of the element fluorine, and for the adoption in the service of science of the electric furnace called after him". His original paper entitled Le Fluor et ses Composés is available online. The French scientist, André Ampère coined the name fluorine in 1812. The name originates from the Latin word 'fluo' meaning flow. Other famous scientists such as Humphry Davey had ‘seen’ fluorine but did not manage to successfully isolate it, as it reacted with their apparatus.
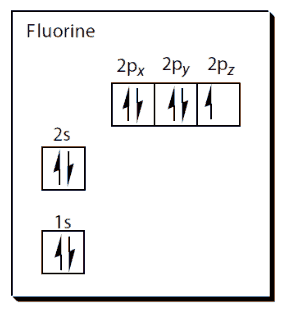
Fluorine is a pale-yellow diatomic gas (F2) with a m.p. of -220°C, a b.p. of -180°C and a density of 1.8 x 10-3 g cm-3. Fluorine has two electrons in its first shell and seven in its outer shell, making its electron configuration 1s2 2s2 2p5.
Fluorine is the most electronegative element, with a value of 4.0 on the Pauling scale, and as it attracts electrons more powerfully than any other element it always has an oxidation number of -1.
Fluorine is a highly dangerous element to handle, being toxic, oxidising and corrosive. It causes severe chemical burns on contact with the skin, and like the other halogens ‘smells’ like swimming pools. Fluorine chemistry is of such importance that it has even got its own journal.
Fluorine reacts with all the halogens and the product depends on the condition and the stoichiometric ratio of reactants.
| Halogen | conditions | Reactions producing interhalogens |
|---|---|---|
| Chlorine | 225°C | Cl2(g) + F2(g)  2ClF(g) 2ClF(g)Cl2(g) + 3F2(g)  2ClF3(g) 2ClF3(g) |
| Chlorine | 350°C, 225 atm | Cl2(g) + 5F2(g)  2 ClF5 (g) 2 ClF5 (g) |
| Bromine | standard | Br2(g)+ F2(g)  2BrF(g) which disproportionates 2BrF(g) which disproportionates3 BrF(g)  Br2(l) + BrF3(l) Br2(l) + BrF3(l)5 BrF(g)  2 Br2(l) + BrF5(l) 2 Br2(l) + BrF5(l) |
| Bromine | 150°C excess fluorine | Br2(g) + 5 F2 (g)  2 BrF5 (l) 2 BrF5 (l) |
| Iodine | -45°C in CCl3F (solvent) | I2(g) + F2(g) 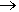 2 IF(g) which disproportionates 2 IF(g) which disproportionates5 IF(g) 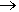 2 I2 (s) + IF5(l) 2 I2 (s) + IF5(l)
|
While trying to isolate Fluorine several 19th-century scientists, the so called Fluorine Martyrs, were blinded or even killed in the attempt to isolate such a reactive element. Earlier unsuccessful attempts included electrolysing hydrofluoric acid, which yielded oxygen and hydrogen rather than fluorine and hydrogen, due to the fact that fluoride ions are less able to give up their charge than the oxide ions. It was in 1886 after seventy-four years of slow progress that Henri Moissan successfully managed to isolate fluorine. He discovered that when potassium bifluoride (KHF2) was combined with anhydrous hydrogen fluoride the mixture conducted electricity, and produced fluorine. This is because potassium bifluoride allows the hydrogen fluoride to dissociate into its ions as follows: HF 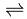 H+ + F-, and the hydrogen fluoride present then dissolves this so that the overall equation is as follows: 3HF
H+ + F-, and the hydrogen fluoride present then dissolves this so that the overall equation is as follows: 3HF 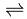 H2F+ + HF2− , which is similar to the auto-protolysis of water, 2H2O
H2F+ + HF2− , which is similar to the auto-protolysis of water, 2H2O 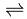 H3O+ + OH-
H3O+ + OH-
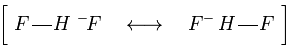
Resonance structure of the bifluoride.
Today fluorine is produced on an industrial scale by electrolysing at 8-12 V a 1:2 mixture of potassium fluoride (KF) and anhydrous hydrogen fluoride (HF) at 70-130°C. The mixture is housed in a steel container that acts as the cathode, and a graphite block is present and acts as the anode. When potassium fluoride and hydrogen fluoride come into contact with each other a spontaneous reaction occurs that produces potassium bifluoride, which, as previously mentioned, allows the mixture to conduct electricity and results in the production of fluorine and hydrogen gas. These two gases must not be allowed to come into contact, as fluorine reacts explosively with hydrogen under all conditions.
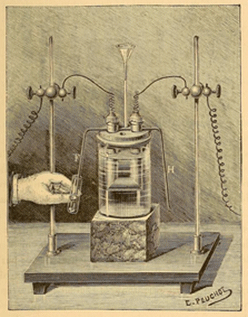
Old apparatus for making fluorine gas.
Water must be removed as soon as it is formed because fluorine will oxidise water to oxygen.
2 F2 + H2O 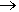 4 HF + O2
4 HF + O2
The hydrogen gas liberated at the cathode must be kept separate from the fluorine gas liberated at anode. This separation is achieved using a diaphragm and this prevents a violent explosion between the hydogen and fluorine. Graphite, an allotrope of carbon, is often as an electrode in electrolysis. Graphite cannot be used as the anode as fluorine will react at the surface to form carbon monofluoride also known as graphite fluoride with an empirical formula CF. It has been found that powdered coke and copper (ungraphitized carbon) is a good alternative for the anode.
Fluorine is extremely reactive, and as it will attack glass and metals careful choice of reaction vessel is needed. Moissan used a platinum U-shaped tube to extract the fluorine Gas. Due to the high cost of platinum, either copper or the alloy Monel metal (Cu/ Ni) can be used as suitable materials for reaction vessels. The vessel’s surface are also covered with a layer of fluoride film to slow down further attack by the highly reactive fluorine.
Fluorine is a very powerful oxidising element with a standard electrode potential of +2.87 V. Other oxidising agents have less positive standard electrode potentials, e.g. hydrogen peroxide (H2O2) +1.77 V and potassium manganate(VII) KMnO4 +1.70 V, and chlorine (Cl2) +1.36 V. The more positive the standard electrode potential is the better the oxidising agent. Fluorine oxidises all non-metals except helium (He), nitrogen (N2), neon (Ne) and argon (Ar). Fluorine even reacts with the usually unreactive and precious metals; gold (Au) and platinum (Pt). All the halogens, except fluorine, dissolve in water making aqueous solutions. Fluorine is different, because it oxidises water to oxygen whilst is reduced to hydrogen fluoride. Fluorine can also react with water to produce ozone (O3).
2 F2 (g) + 2 H2O (l)  4 HF (aq) + O2 (g)
4 HF (aq) + O2 (g)
3 F2 (g) + 3 H2O (l) 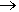 O3 (g) + 6 HF (g)
O3 (g) + 6 HF (g)
When fluorine oxidises other elements it tends to oxidise them to their highest possible oxidation states, e.g. PF5, SF6, UF6, XeF6 and IF7. The small size of a fluorine atom means that many fluorine atoms can fit around a central atom. SF6 is used in electrical insulation and has the opposite effect to helium on the human voice when inhaled, that is, it makes the voice sound deeper. UF6 ("Molecule of the Month" August 2002) is used to separate radioisotopes.
The surprisingly weak F-F bond (158 kJ mol-1) compared to the other halogens is a result of fluorine having such small atoms, which brings the lone pairs close enough to experience electrostatic repulsion as indicated by the arrows.
Fluorine is not a very abundant element but it is one which is widely distributed in different minerals as its anion, fluoride (F-). Some of the main minerals containing fluoride are fluorspar (CaF2) also known as fluorite, cryolite (Na3AlF6) used as a solvent in the electrolysis of aluminium oxide and fluorapatite 3Ca3(PO4)2.Ca(ClF) found in teeth. It was possible to get flouride treatment from the 1970-80s at a dentists to help strengthen the enamel of teeth.
| Mineral | Formula | Property | Crystal |
|---|---|---|---|
| Fluorspar (fluorite) | CaF2 | Fluorescence when heated |  |
| Fluoroapatite | Ca3(PO4)2.CaF2 | Major source of phosphorus |  |
| Cryolite | Na3AlF6 | Solvent used to be used in the electrolysis of aluminium from its ore bauxite (Al2O3) |  |
| Griceite | LiF | discovered 1989 |  |
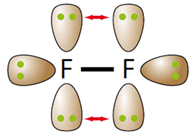
On a trip to the Natural History Museum in London try to find the display of minerals which glow under UV light. The picture below shows the effect of UV light on fluorite.
Fluorescence in crystals under UVm light
The mineral fluorspar which is calcium fluoride (CaF2) reacts with sulfuric acid (H2SO4) to produce calcium sulfate (CaSO4) and hydrogen fluoride (HF). The hydrogen fluoride is electrolysed in the present of potassium fluoride (KF) to form potassium bifluoride (KHF2) to make elemental flourine as described earlier.
CaF2 + H2SO4 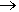 CaSO4 + 2 HF
CaSO4 + 2 HF
KF + HF  K[HF2]
K[HF2]
Fluorine containing compounds are used also as fluxes when extracting iron and aluminium from its ore. The main ore for iron is hameatite (Fe2O3) and the main ore for aluminium is bauxite (Al2O3). Calcium fluoride is used to lower the melting temperature of steel but also to reduce its viscosity. Cryolite has been used to lower the temperature of refined bauxite, aluminium ore, in the production of aluminium. The cryolite was originally extracted from mineral deposits in Greenland. The extraction of mineral resources in Greenland by mining companies will have benefits and risks which will need to be carefully weighed up by this former Danish colony.
The pharmaceutical industry is making ever increasing use of fluorine. In the last thirty years approximately 15% of new drugs contain at least one fluorine atom. Since carbon-fluorine bonds are more stable (544 kJ mol-1) than carbon-hydrogen bonds (413 kJ mol-1), the drug takes longer to metabolise and break down in the body. This greater stability is useful as it allows longer times between doses of drugs. Carbon-fluorine bonds are more hydrophobic than carbon-hydrogen bonds, the result of this being that the drug is more readily dissolved in body fats.
The many uses found for fluorine compounds cover a broad spectrum of medical conditions as illustrated by the examples below. For those interested a more complete list can be found via this link.
| Fluorine containing molecule | Name (Use) |
|---|---|
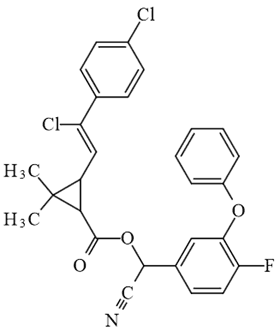 | Flumethrin (insecticide) |
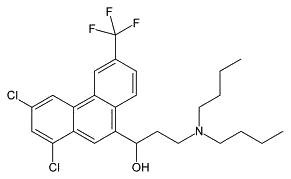 | Halofantrine (antimalarial) |
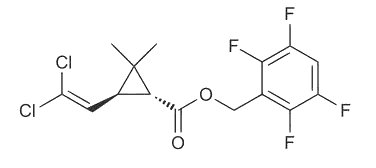 | Transfluthrin (moth killer) |
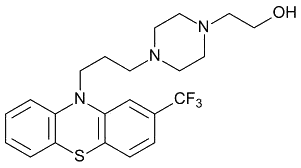 | Prolixin (anti-psychotic) |
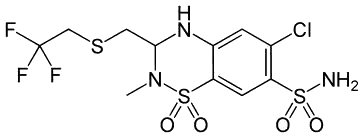 | Polythiazide (antihypertensive) |
In addition to using fluorine in compounds, an isotope of fluorine is finding cancers in patients. The usual and only stable isotope of fluorine is 19F (fluorine-19). Fluorine-18, a positron emitting unstable isotope, used in PET scan, (positron emission tomography). The decay chain of fluorine-18 is F-18 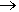 O-18 + positron. Fludeoxyglucose (FDG) is a glucose containing compound that has one hydroxy group replace by a fluorine-18 atom. When injected into the bloodstream of a patient, the FDG is taken up more rapidly by malignant cancerous tumour cells than normal cells. The positrons emitted by fluorine-18 can be detected, and hence tumours can be found using this method of scanning.
O-18 + positron. Fludeoxyglucose (FDG) is a glucose containing compound that has one hydroxy group replace by a fluorine-18 atom. When injected into the bloodstream of a patient, the FDG is taken up more rapidly by malignant cancerous tumour cells than normal cells. The positrons emitted by fluorine-18 can be detected, and hence tumours can be found using this method of scanning.
The Royal Society of chemistry continues to produce world class chemistry resources. For their series on fluorine there is a podcast and a video.
![]()