Although early archaeological evidence in the form of spoon-shaped incense burners have been found from the Old Kingdom Ancient Egypt, no chemical evidence of the exact resin used exists. The Evershed group at Bristol University has chemically characterised frankincense from the archaeological record at Quasr Ibrim, Egypt.
They characterised the resins by a combination of gas chromatography and mass spectrometry (GC/MS) using solvent solubilisation, derivatisation and pyrolysis techniques.
Pentacyclic triterpenoid components present in modern frankincense resin were determined my mass spectrometry (Fig. 1). Especially characteristic are the a- and b- boswellic acids and the corresponding O-acetyl derivatives which dominate the mass spectra of both modern and ancient frankincense (Fig. 2).
These data were supported by detection of 24-noroleana-3,12-diene and 24-norursa-3,12-diene, which are the most abundant components of the Curie-point (610 deg. C) pyrolysates of the extracted archaeological resin and reference frankincense. The boswellic acids and their O-acetyl derivatives are the major constituens of the fresh aromatic gum resins from Boswellia trees.
They characterised the resins by a combination of gas chromatography and mass spectrometry (GC/MS) using solvent solubilisation, derivatisation and pyrolysis techniques.
Pentacyclic triterpenoid components present in modern frankincense resin were determined my mass spectrometry (Fig. 1). Especially characteristic are the a- and b- boswellic acids and the corresponding O-acetyl derivatives which dominate the mass spectra of both modern and ancient frankincense (Fig. 2).
These data were supported by detection of 24-noroleana-3,12-diene and 24-norursa-3,12-diene, which are the most abundant components of the Curie-point (610 deg. C) pyrolysates of the extracted archaeological resin and reference frankincense. The boswellic acids and their O-acetyl derivatives are the major constituens of the fresh aromatic gum resins from Boswellia trees.
Figure 1: Mass spectrometry of triterpenoids of ancient frankincense. Electron ionisation (70 eV) mass spectra of the trimethylsilyl derivatives of b-boswellic acid (upper) and O-acetyl-b-boswellic acid (lower) obtained from the GC/MS analysis of ancient frankincense. Insets: structures showing the origins of the fragment ions used to plot characteristic mass chromatograms (Fig. 2).
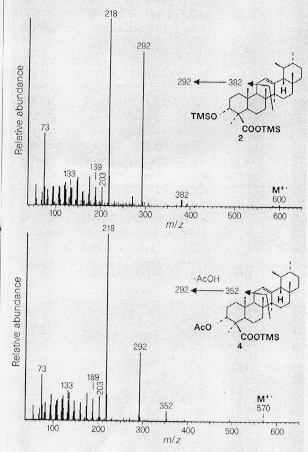
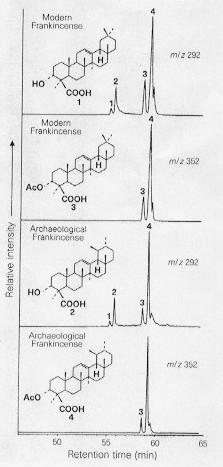
Figure 2: Comparison of ancient and modern frankincense. Partial GC/MS mas chromatograms of the trimethylsilylated solvent-soluble fractions of a sample of reference frankincense and of resin recovered from Quasr Ibrim. The structures shown are: 1, a-boswellic acid; 2, b-boswellic acid; 3, O-acetyl-a-boswellic acid; 4, O-acetyl-b-boswellic acid. The m/z 352 mass chromatograms characterise O-acetyl derivatives, the major components of both the ancient and modern resin. The m/z 292 chromatograms show the similarity of the distributions of the O-acetyl and free alcohol forms. Inset: structures of compounds in their natural (underivatised) forms.