![]()
Hydrazine
Rocket fuel, spandex suits, power stations and car air-bags!
![]()
Paul May
University of Bristol
![]()
Molecule of the Month January 2014
Also available: HTML version.
![]()
|
HydrazineRocket fuel, spandex suits, power stations and car air-bags!
Paul May
Molecule of the Month January 2014
|
 |
Yes, it's either used for those applications directly or is involved in their manufacture.
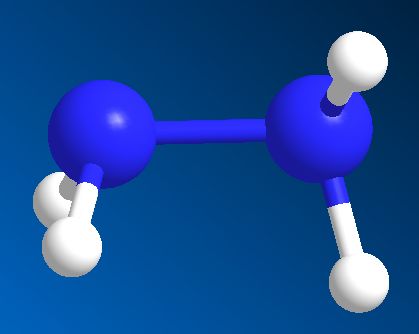
Hydrazine is an extremely toxic and dangerously unstable liquid that smells a bit like ammonia - probably because it's made from two ammonia molecules joined together (with the loss of H2). Its formula is N2H4 and its structure resembles somewhat the skewed structure of hydrogen peroxide.
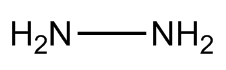 |
 |
|
Hydrazine | ||
 How is it made?
How is it made?It was first made in 1889 by the German chemist Theodor Curtius, but his method was rather inefficient. Nowadays, there are several methods used to make hydrazine industrially, including the Olin-Raschig process which ws developed by another German chemist, Friedrich Raschig (photo, right), in 1907. In this process, sodium hypochlorite (the active ingredient in bleach) is mixed with ammonia at 5ºC to form chloramine and sodium hydroxide. This mixture is then rapidly added to anhydrous ammonia under pressure and at 130ºC to produce hydrazine and water, plus salt as a waste product.
NaOCl + NH3  NH2Cl + NaOH
NH2Cl + NaOH
NH2Cl + NaOH + NH3(anhyd.)  N2H4 + H2O + NaCl
N2H4 + H2O + NaCl
It has two main properties that make it useful. First, it's a strong reducing agent, with often the only by-products being water and nitrogen. As such it is used to reduce plutonium oxides in nuclear waste back to Pu metal, and is added to the water-cooling systems of power stations to stop the pipes corroding/oxidising. It does this by scavenging dissolved oxygen, which would otherwise attack the metal.
N2H4 + O2 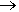 N2 + 2H2O.
N2 + 2H2O.
It's also used as a precursor for some organic syntheses, and in applications as diverse as fuel cells, a catalyst for polymerisation, solder fluxes and in photographic developing fluid. It's also used to make some of the precursors required in the polymerisation reaction that produces spandex (Lycra) fibres, something for which sportspeople, glam-rockers, and wannabe superheroes should be grateful.



Superman in spandex.....Wrestlers in spandex.....but spandex doesn't suit everyone!
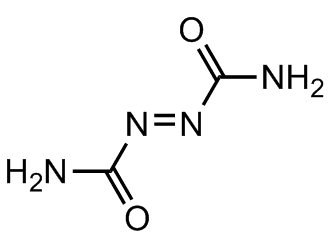
However, its main industrial use is as a precursor for making blowing agents. These are gases which are blown through a liquid which causes it to foam, and then harden into a lightweight solid, an example being expanded polystyrene. The two main blowing agents made from hydrazine are azodicarbonamide and azobisisobutyronitrile. Azodicarbonamide is used to produce foamed plastic, because when heated it decomposes into N2, CO, CO2 and NH3 which then get trapped as bubbles in the polymer. Azobisisobutyronitrile is also used as a foamer in plastics and rubber, but is also used as a radical initiator in polymerisation reactions because it readily expels N2 gas leaving two CN-substituted cyclopropane radicals behind to begin the free-radical chain reaction.
 |
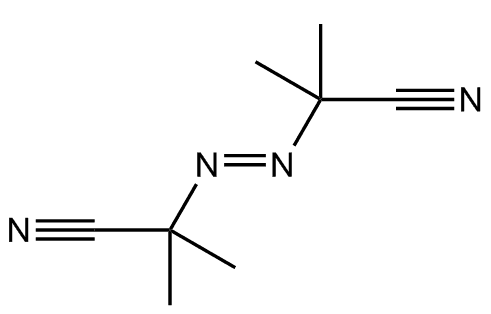 |
Azodicarbonamide |
Azobisisobutyronitrile |
Similarly, hydrazine is used to make sodium azide by reaction with sodium nitrite:
NaNO2 + N2H4 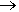 NaN3 + H2O2
NaN3 + H2O2
and the sodium azide is the explosive that's used in car air-bags and aircraft escape-chutes since it decomposes rapidly on heating to produce lots of N2 gas.
2NaN3 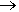 2Na + 3N2
2Na + 3N2


Left: An airbag powered by sodium azide is tested with a crash-test dummy. Right: An Me-163, the World's first rocket-powered airplane, powered by hydrazine.
That application comes about due to the other important property of hydrazine: it burns very exothermically in the presence of oxygen (or oxygen-containing compounds) generating a lot of hot gases, which can be used to produce the exhaust thrust for a rocket. This was first exploited in WW2, when the German Me163B Komet became the world's first rocket-powered fighter plane. Various fuels were used including a 1:1 mixture of hydrazine and water (known as hydrazine monohydrate), "C Stoff" (57% methanol, 30% hydrazine, 13% water) and "T Stoff" (80% H2O2, 20% water), which were kept in separate tankers at opposite ends of the airfield and were always at least half a mile apart. These were highly explosive mixtures, so it's no surprise that more German pilots were killed in this plane by fuel leakages and/or explosions than were shot down by the Allies (see MOTM page on hydrogen peroxide).
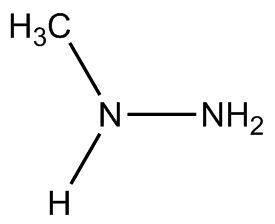
To lower (slightly) the risk of unwanted explosion, other variants of hydrazine have been used as rocket fuels, such as monomethylhydrazine (MMH), (CH3)NH(NH2) and unsymmetrical dimethylhydrazine (UDMH), (CH3)2N(NH2). These are often mixed with dinitrogen tetroxide (N2O4) with the advantage being that no ignition source is needed - the two compounds spontaneously combust on contact. These mixtures are normally used in military, orbital, and deep-space rockets because both liquids are storable for long periods at reasonable temperatures and pressures, but not generally for civilian spacecraft due to the toxicity and explosion risks.
 |
 |
MMH | UDMH |
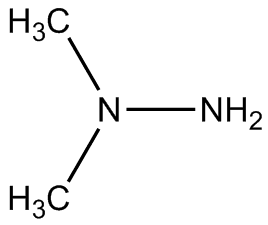
More often nowadays, hydrazine is used for manoeuvring thrusters or for slowing spacecraft down on re-entry. Hydrazine-fuelled thrusters were used to land spacecraft on Mars, including the Viking spacecraft in the 1970s, the Phoenix lander (May 2008) and the Curiosity rover (August 2012). In these thrusters, an iridium catalyst, supported on an inert alumina matrix, decomposed the hydrazine to produce ammonia, nitrogen and hydrogen gases. The reactions produce a very large quantity of gas from a small amount of liquid hydrazine, and the pressurised hot gas is expelled from the spacecraft producing thrust.
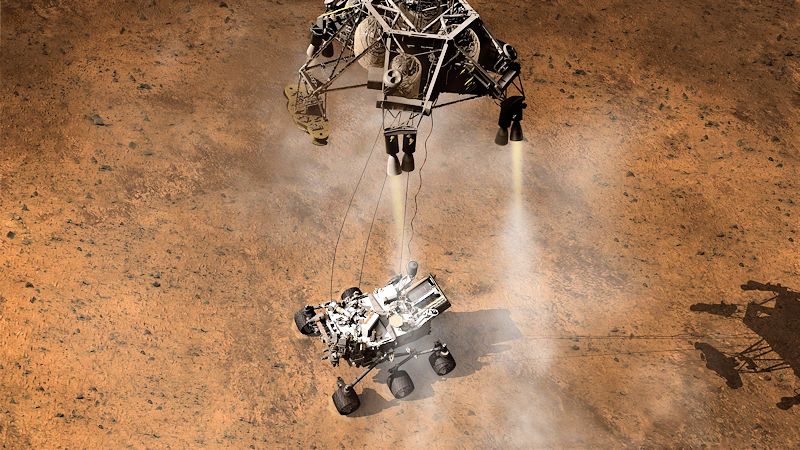
Artist's impression of Curiosity firing its hydrazine thrusters when landing on Mars in 2012.
![]()
![]()