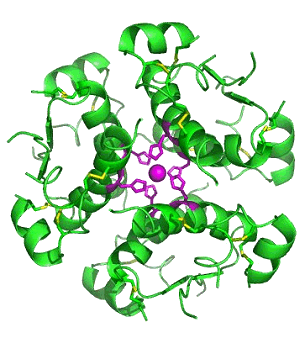
Computer-generated image of six insulin molecules
assembled in a hexamer (from Ref.[1]).
3D structure file
|

INSULIN
The hormone that converts sugar
in the blood into asource of energy
for our body's metabolic processes

Maria Kyriakou
Bristol University
Molecule of the Month - July 2010

|
![Image taken from ref.[2]](Thankful.gif)
Image taken from Ref.[2]. |
"Laughter is the best medicine- unless you're diabetic, then insulin comes pretty high on the list" - Jasper Carrott [3]
So, what is a hormone, really?
Hormones are chemical components that are produced in one part of the body and function at an entirely different one [4]. They include proteins, peptides and lipids, existing usually as precursor molecules [5]. In addition hormones have various functions such as the regulation of metabolism and its development. A major role is to act as messengers and carry information around tissues in the body by binding receptors on cell surfaces or within a cell [5]. Receptors are proteins on cell surfaces or within them with available sites for the signalling ligand molecule to bind on. The receptor's major role is to act as the conductor for hormones and drugs to reach the body and the target they require. One of the main peptide hormones studied through years due to its importance and necessity is insulin, whose structure, synthesis and function are discussed further below.
What does insulin do?
Insulin is a hormone that is central to regulating glucose metabolism in the body to produce energy. Insulin causes cells in the liver, muscle, and fat tissue to take up glucose from the blood, which it then converts to glycogen which is stored in the liver and muscle. When insulin is absent, glucose is not taken up by body cells and the body begins to use fat as an energy source.
What about insulin's structure? It seems to be very complicated.
Insulin is a peptide hormone composed of 51 amino acids and has a molecular weight of 5808 Da. It is produced in the islets of Langerhans in the pancreas. The name comes from the Latin insula for "island". Insulin's structure varies slightly between species of animals. Insulin from animal sources differs somewhat in "potency" in humans because of those variations. Pig insulin is especially close to the human version. Insulin was the first protein whose amino acid sequence was identified and the first to be synthesized. It is initially synthesized as a precursor molecule known as proinsulin and on cleavage it gives two peptides chains, one comprising of 21 amino acids and the other of 30 amino acids, being mostly conserved in humans and animals [6] (see Figure 1). The two chains are joined together by two disulfide bonds between two cysteine residues, where a disulfide bond is the linkage bond between two sulfurs as shown below.
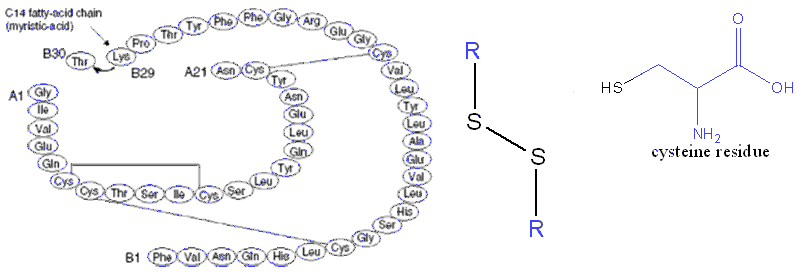
Figure 1. The two chains comprising the insulin structure, A and B, indicating also a disulfide bond between cysteines (R represents the molecular fragment coloured in blue), since they are very important in stabilizing the two chains together. Chain structure from Ref. [8].
Espinal says in his book [6] that X-ray analysis in 1926 showed that insulin exists as hexameric rhombohedral crystals and that its dimeric form has a relative molecular weight of 12000. Each hexamer is either bound to two zinc ions or to four zinc ions. There is also a calcium binding site, and finally any other available sites are occupied by water molecules or another ligand [7]. The hexamer and some of its binding sites are indicated by Figure 2.
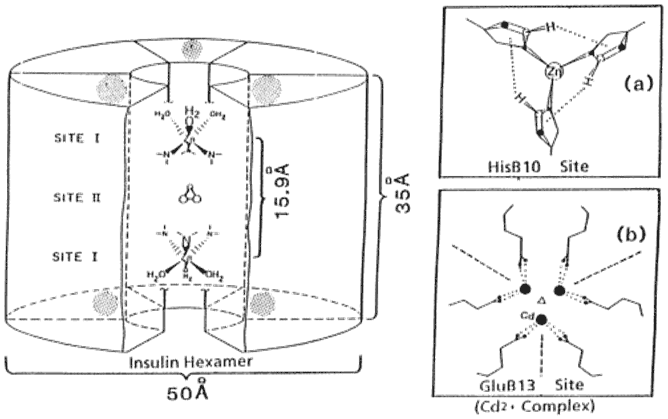
The insulin hexamer with two of the main binding sites shown next to it. From Ref.[7].
In addition, it is very important to realize that most of hormone functions are more potent once the 3D structure is obtained. Consequently modifications on any of the amino-acids of the two chains or any conformational changes alter the activity as well [9]. Finally, it is worth mentioning that achievements to build up newly modified structures have been done, as reported by Schuttler and Brandenburg [10], by cross-linking, for example, insulin dimers together, since such alterations can be very helpful in obtaining more information on insulin-secretion mechanisms and action.
Banting, Best, Macleod - The discoverers of insulin!
Starting from the very early times, in around 1889, Minkowski and Von Mering, used pancreas from dogs to produce and study the different effects that diabetics exhibited, since the pancreas is the major tissue where insulin is involved. Minkowski's conclusions were mainly that the pancreas undergoes a secretion process, and that these secreted species could regulate the metabolic pathways of carbohydrates [6], such as the oxidation of glucose, glucose generation and even the metabolism of lipids.
Around 1920, Frederick Banting was working as a surgeon in London. On reading an article from Minkowiski, he suggested to the Professor of Physiology J.J.R. Macleod at the University of Toronto that the problems existing, as far as the pancreas was concerned, had to do with internal secretion abnormalities. Given permission, Banting worked with a science student, Charles Best, who extracted pancreas from dogs and analyzed blood sugar levels, especially in diabetics. By 1922, the first papers on pancreas secretion were published and in 1923 Banting and Macleod were awarded the Nobel prize for discovering insulin. Over the next years, insulin was produced in large amounts at the University of Toronto earning them great profits, and in 1971 the International Diabetes Federation published an account based on insulin discovery [11].

Figure 3. A) Frederick Banting [12]
|

B) Charles Best [13]
|

C) J.J.R Macleod [13]
|
How is insulin secreted from the pancreas?
Insulin is secreted from the human pancreas and its release is mainly triggered when large concentrations of glucose are detected in the blood after a meal. Its main role is thus the uptake of glucose by increasing the rate of the glycolytic pathway - the process where glucose is converted to other carbohydrates that are used in the urea cycle or for fatty-acids metabolism.
Glucose initially enters the β-pancreatic cells by the help of transporters known as GLUT 2 that carry the sugar across the cell membrane, so the action of glucose seems to be responsible for most of the following changes undertaken. At the beginning, an increase in the ATP/ADP ratio is observed [14], the energy currency involved in all metabolic pathways. This rise selectively affects ATP-sensitive K+ ion channels by closing them and thus preventing K+ passage across the cell membrane (Figure 4). This decreases the charge difference that already exists between the inside and outside of the membrane - the effect known as depolarization. At the same time, an increasing electrical conductivity is observed, driving the opening of voltage-sensitive calcium-ion channels [14,15]. The opening of this channel allows the entrance of Ca2+ ions into the membrane which initially bind certain proteins such as calmodulins or synaptotagmins. As a result, this drives the densely packed vesicles of insulin to open and release insulin, by emerging through the membrane of the β-pancreatic cells [15].
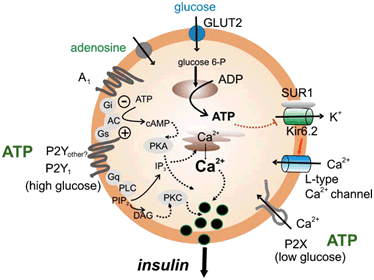
The insulin secretion mechanism in detail. From Ref.[16].
Interesting information on insulin secretion from their chemistry side:
- Receptors, known as purinergic receptors, are responsible for ATP to act through them and trigger the observed Ca2+ ion release [16].
- Very long-chain fatty-acids, such as arachidonic acid made from 20 carbons, may regulate the action of the potassium ion channels involved or the calcium ion release [17].
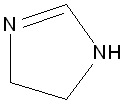 Imidazolines, with functional group shown, act as binding sites for essential proteins in the human brain [18] and influence the secretion of insulin at the same time by acting on KATP-channels, the most interesting bit being that regioisomers and enantiomers can have entirely different effects [19].
Imidazolines, with functional group shown, act as binding sites for essential proteins in the human brain [18] and influence the secretion of insulin at the same time by acting on KATP-channels, the most interesting bit being that regioisomers and enantiomers can have entirely different effects [19].
How does insulin work?
All insulin's actions peak after feeding, whereas on starving, the hormone's main role is glucagon production. There are three main sites to consider for insulin's activities, those being the liver, muscle and adipose tissue.
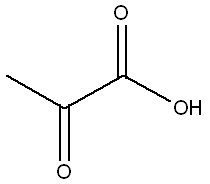 Starting from blood glucose levels upon the fed state, insulin has the main role to increase the rate of glucose oxidation (glycolysis) in the liver and muscle while at the same time it converts glucose concentrations to glycogen (the form in which glucose can be stored in the body)[6]. These processes are supported by an increase in the number of glucose transporters to the cell membrane which have a significant role, as described above, as well as by a variety of phosphorylation and dephosphorylation processes (Scheme 1) for activation and deactivation of enzymes (biological catalysts). In order for an enzyme to be phosphorylated, a PO32- group is donated to it by adenosine triphosphate, ATP. The resulting increase in the rate of glycolysis leads to pyruvate (see structure, right) production which is then converted to Acetyl-CoA, one of the main components in biological processes, since it supplies muscles with energy and it is used in lipid synthesis in the liver and adipose tissue [6]. Finally, it is worth mentioning that since glucose oxidation is stimulated by insulin, the reverse process in the liver, gluconeogenesis, is inhibited.
Starting from blood glucose levels upon the fed state, insulin has the main role to increase the rate of glucose oxidation (glycolysis) in the liver and muscle while at the same time it converts glucose concentrations to glycogen (the form in which glucose can be stored in the body)[6]. These processes are supported by an increase in the number of glucose transporters to the cell membrane which have a significant role, as described above, as well as by a variety of phosphorylation and dephosphorylation processes (Scheme 1) for activation and deactivation of enzymes (biological catalysts). In order for an enzyme to be phosphorylated, a PO32- group is donated to it by adenosine triphosphate, ATP. The resulting increase in the rate of glycolysis leads to pyruvate (see structure, right) production which is then converted to Acetyl-CoA, one of the main components in biological processes, since it supplies muscles with energy and it is used in lipid synthesis in the liver and adipose tissue [6]. Finally, it is worth mentioning that since glucose oxidation is stimulated by insulin, the reverse process in the liver, gluconeogenesis, is inhibited.
At low carbohydrate concentrations, fatty-acid breakdown in the liver is reduced, whereas synthesis is facilitated since triacylglycerols in the adipose tissue are converted to long chain fatty acids. This is favoured due to the larger availability of the responsible synthesizing enzyme, lipoprotein lipase [20].
Finally, secondary actions of insulin include stimulation of protein synthesis [20] as well as increased blood flow, vasodilatation, and hypotension [21].
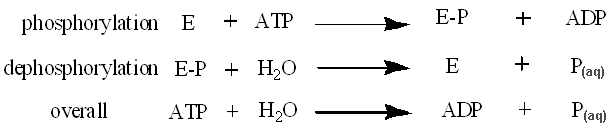
Scheme 1. The reactions of phosphorylation and dephosphorylation of enzymes, part of almost all biological processes. E=enzyme, P = phosphate.
All of the actions of insulin are performed via insulin receptors, known as tyrosine kinase receptors. These are 2-subunit receptors and contain both an extracellular domain for insulin to bind as a ligand as well as an intracellular part, insulin protein kinase [22] where all phosphorylation events take place. As with most polypeptide hormones, upon binding, conformational changes are undertaken on the two subunits and a series of phosphorylation events proceed through, leading to more of the actions hormones perform (Figure 5). Internalization of insulin upon binding keeps it in the cell followed by degradation at the end [23]. In addition, it is worth mentioning that all of these actions and signalling pathways are accompanied by the formation of second messengers such as cyclic adenosine phosphate, cAMP. Second messengers are molecules that disperse information around tissues. The decrease in the concentration of cAMP is the main cause for insulin's activities and this is because of insulin suppressing the precursor molecule, adenylate cyclase, from which the second messenger is synthesized [24].
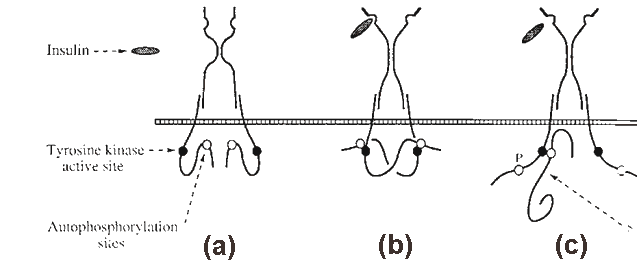
Figure 5. The tyrosine kinase receptor indicating the external and internal sites it consists of,
as well as the conformational changes undertaken. From Ref.[22].
Interesting tips to make your insulin function better:
- Upon exercise, insulin is acting more efficiently on glucose breakdown and on lipid metabolism [25]. Just run to the gym now!
- Water extracted from cinnamon, grown in India, was proved to elevate insulin activity on glucose oxidation and glycogen synthesis in storing glucose [26].
- Metals of group 12, Zn, Cd and Hg, stimulate the rate of glycolysis by affecting the signalling pathway, and HgCl2, specifically, favours the action of glucose transporters by altering the cell membrane permeability [27].
- Zn (II) ions and their complexes facilitate lipogenesis, the formation of fatty acids, and the translocation of glucose on cell membrane of adipose tissue [28].
Is diabetes mellitus the disease of the future?
![Diabetes cartoon, from Ref.[2].](meds.gif) The main disease which greatly involves insulin is known to be diabetes mellitus. The term 'mellitus' can be translated as honey or sweet-tasting urine and, as Thomas Willis observed in the 17th century, "diabetics p**s a great deal"! Type 1 diabetes is the insulin-dependent diabetes (IDDM) whereas type 2, is the non-insulin dependent (NIDDM). Type-1 diabetes, being the most famous, exists due to damaged β-pancreatic cells and thus their unavailability to excrete insulin in the normal way described above. It can be considered as a genetic problem to be inherited, or may be because of environmental effects [29]. Due to this insulin deficiency, glucose transporters are suppressed and glycogen synthesis is limited, whereas synthesis of glucose and protein degradation are facilitated [30], all being the opposite functions that normal insulin secretion produces. Usually it can be treated by replacing the pancreatic tissue through transplantation [31] since it is greatly damaged, but most people nowadays perform injections or use insulin pumps. On the other hand, in type-2 diabetes the pancreatic β-cells function quite perfectly, sometimes over-secreting insulin, but tissues around remain unaffected by it. It is also related to obesity. Finally gestational diabetes is another type of diabetes appearing only to pregnant women though, as a result of problematic insulin secretion [29].
The main disease which greatly involves insulin is known to be diabetes mellitus. The term 'mellitus' can be translated as honey or sweet-tasting urine and, as Thomas Willis observed in the 17th century, "diabetics p**s a great deal"! Type 1 diabetes is the insulin-dependent diabetes (IDDM) whereas type 2, is the non-insulin dependent (NIDDM). Type-1 diabetes, being the most famous, exists due to damaged β-pancreatic cells and thus their unavailability to excrete insulin in the normal way described above. It can be considered as a genetic problem to be inherited, or may be because of environmental effects [29]. Due to this insulin deficiency, glucose transporters are suppressed and glycogen synthesis is limited, whereas synthesis of glucose and protein degradation are facilitated [30], all being the opposite functions that normal insulin secretion produces. Usually it can be treated by replacing the pancreatic tissue through transplantation [31] since it is greatly damaged, but most people nowadays perform injections or use insulin pumps. On the other hand, in type-2 diabetes the pancreatic β-cells function quite perfectly, sometimes over-secreting insulin, but tissues around remain unaffected by it. It is also related to obesity. Finally gestational diabetes is another type of diabetes appearing only to pregnant women though, as a result of problematic insulin secretion [29].
Insulin resistance - Obesity and differences between the two sexes.
Insulin resistance appears to be a main condition for type-2 diabetes, defined as "reduced responsiveness to normal circulating levels of insulin" [33]. This is directly related to obesity, and insulin resistance arises mostly due to adipose tissue abnormalities. As a result other tissues are now more exposed to fats and thus lots of them are concentrated at one position resulting to obesity after an extensive exposure [34]. Taking the opposite condition into consideration, experiments have shown that obese people have a greater risk of becoming diabetics and using statistics made in England, as an example, it is thought that the current 2.35 million diabetics will increase to 2.5 million in the near future [35]. Figure 6 indicates some of the main alterations that happen due to insulin resistance in the liver and muscles.
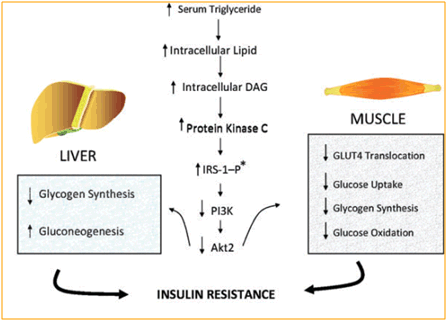
Figure 6. The effects in muscle and liver build up because of insulin resistance
in diabetes 2, thus leading to obesity. From Ref. [33].
Furthermore it is very interesting to have knowledge on how insulin resistance varies between men and women, since a variety of factors may affect the way males and females are affected by type-2 diabetes.
Mittendorfer [36] suggests that generally females are more insulin resistant compared to men and this is mostly related to the metabolic pathways that concern the release and actions of hormones in the two sexes. First of all, inheritance of the diabetic syndrome is more intense among women rather than men, especially from the mother's side. In addition, as far as obesity is concerned and as mentioned above, overweight people are in a larger risk to adopt the known 'metabolic syndrome', being common among both sexes although women's risk is high because they concentrate lots of lipid in localised areas of the body. Finally, exercise is generally beneficial, but for females it is essentially to be accompanied by diet and weight loss so as to reduce the risks of type-2 diabetes more. As a result, women seem to be in a higher risk of becoming diabetics and so it is partly in our hands to prevent the diabetes "monster" from attacking.
Bibliography
- http://en.wikipedia.org/wiki/Insulin
- Islets of humor from Defeat Diabetes
- Jasper Carrot, http://www.brainyquote.com/quotes/quotes/j/jaspercarr281919.html.
- F.F. Bolander, Molecular Endocrinology, 3rd edn, Elseiver Academic Press, London, 2004.
- 5. F. S Greenspan and D. G. Gardner, Basic and Clinical Endocrinology, 7th edn, McGraw-Hill Companies, New York, 2004.
- J. Espinal, Understanding Insulin Action: principles and molecular mechanisms, Ellis Horwood, Chichester, 1989.
- W. Kadima, Role of metal ions in the T-to-R allosteric transition in the insulin hexamer, Biochemistry, (1999), 38, 13443.
- L. B. Chaykin, Insulin detemir and its unique mechanism of action, The Internet J. Endocrinol., (2007), 4, number 1.
- G. G. Dodson, S. Cutfield, E. Hoenjet, A. Wollmer, D. Branderburg, Crystal structure, aggregation and biological potency of beef insulin cross-linked at A1 and B29 by diaminosuberic acid, in Insulin: Chemistry, Structure and Function of Insulin and Related Hormones: Proceedings 2nd Int. Insulin Symposium, Aachen, 4-7 Sep 1979, eds. D. Branderburg and A. Wollmer, de Gruyter, Berlin, New York, 1980, p. 17-26.
- A. Schuttler and D. Brandenburg, Preparation of covalent insulin dimmers, in Insulin: Chemistry, Structure and Function of Insulin and Related Hormones: Proceedings 2nd Int. Insulin Symposium, Aachen, 4-7 Sep 1979, eds. D. Branderburg and A. Wollmer, de Gruyter, Berlin, New York, 1980, p.143- 149.
- M. Bliss, The discovery of insulin, The University of Chicago Press, Chicago, 1982.
- R. Madeb, L. G. Koniaris, S. I. Schwartz, The discovery of insulin, Rochester, New York, connection, Ann. Int. Med., 2005, 143, 907.
- L. Rosenfeld, Insulin: Discovery and Controvery, Clin. Chem., (2002), 48, 2270.
- D. L. Cook and C. N. Hales, Intracellular ATP directly blocks K+ channels in pancreatic β-cells, Nature, (1984), 311, 271.
- B. R. Gauthier and C. B. Wollheim, Synaptotagmins bind calcium to release insulin, Am. J. Physiol.- Endo. M., (2008), 295, E1279.
- I. Novak, Purinergic receptors in the endocrine and exocrine pancreas, Purinergic signalling, (2008), 4, 237.
- Y. Sato and J.C. Henquin, The K+ - ATP channels- independent pathway of regulation of insulin secretion by glucose - In search of the underlying mechanism, Diabetes, (1998), 47, 1713.
- F. Gentili, C. Cardinaletti, C. Vespirini, F. Ghelfi, A. Farande, M. Giannella, A. Piergentili, W. Quaglia, L. Mattioli, M. Perfumi, A. Hudson, M. Pigini, Novel ligands rationally designed for characterizing I-2-imidazoline binding sites nature and function, J. Med. Chem., (2008), 51, 5130.
- P. Jakobsen, P. Madsen, H.S. Andersen, Imidazolines as efficacious glucose-dependent stimulators of insulin secretion, in European Journal of Medicinal Chemistry: Proceedings 17th Int. Symp. Of Medicinal Chemistry, Barcelona, 01-05 Sep 2002, Editions Scientifiques Medicales Elseveir, Paris, (2003), p. 357-362.
- E. A. Newsholme. S. J. Bevan, G. D. Dimitriadis, R. P. Kelly, in Insulin: Molecular Biology to Pathology, eds. F. M. Ashcroft and S. J. H. Ashcroft, IRL Press, Oxford, 1992, p.155.
- C. Sartori, L. Trueb, P. Nicod, U. Scherrer, Effects of sympathectomy and nitric oxide synthase inhibition on vascular action of insulin in humans, Hypertention, (1999), 34, 586.
- K. Siddle, in Insulin: Molecular Biology to Pathology, eds. F. M. Ashcroft and S. J. Ashcroft, IRL Press, Oxford, 1992, p. 191.
- B. Desbuquois and M. C. Postel- Vinay, Receptor-mediated internalisation of insulin, glucagon and growth hormone in intact rat liver - a biochemical study, in Insulin: Chemistry, Structure and Function of Insulin and Related Hormones: Proceedings 2nd Int. Insulin Symposium, Aachen, 4-7 Sep 1979, eds. D. Branderburg and A. Wollmer, de Gruyter, Berlin, New York, 1980. p. 285-292.
- N. M. Pertseva, A.O. Shpakov, S. A. Plenseva, L. A. Kuznetsova, A novel view on the mechanism of action of insulin and other insulin superfamily peptides - involvement of adenylyl cyclase signalling system, Comp. Biochem. Phys. B., (2003) 134, 11.
- F. Shojaee-Moradie, K. C. R. Baynes, C. Pentecost, J. D. Bell, E. L. Thomas, N. C. Jackson, M. Stolinski, M. Whyte, D. Lorell, S. B. Bowes, J. Gibney, R. H. Jones, A. M. Umpleby, Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose, Diabetologia, (2007), 50, 404.
- B. Roffey, A. Atwal, S. Kubow, , Cinamon water extracts increase glucose uptake but inhibit adiponectin secretion in 3T3-Li adipose cells, Mol. Nutr. Food Res., (2006), 50, 1739.
- D. M. Barnes and E. A. Kircher, Effects of mercury chloride on glucose transport in 3T3- Li adipocytes, Toxicol. in Vitro, (2005), 19, 207.
- Y. Yoshikawa, E. Ueda, Y. Kojima, H. Sakurai, The action mechanism of Zn(II) complexes with insulinomimetic activity in rat adipocytes, Life Sci., (2004), 75, 741.
- M. I. Harris and P. Zimmet, in International textbook of diabetes mellitus, eds. K. G. M. Alberti, P. Zimmet, R. A. Defronzo, Wiley, Chichester, 2nd edn., 1997, vol. 1, p. 9.
- P. J. Watkins, P. L . Drury, S. L. Howell, Diabetes and its management, 5th edn, Blackwell Science Ltd, Oxford, 1996.
- F. Calcinaro, D. L. Wegmann, K. J. Lafferty, in Molecular Biology of Diabetes, eds. B. Daznin, D. LeRoith, N. J. Totowa, Humana Press, 1994, vol. 1, p. 69.
- S. D. Sanders, Endocrine and reproductive systems, 2nd edn, Mosby, London, 2003.
- G. Wolf, Role of fatty acids in the development of insulin resistance and type-2 diabetes mellitus, Nutr. News, (2008), 66, 597.
- M. S. Westerterp, R. P. Mensink, Traces in metabolism and nutrition, Physiol. Behav. (2008), 94, 155.
- L. S. Aucott, Influence of weight loss on long-term diabetes outcomes, in Proceedings of the Nutrition Society: Summer Meeting of the Nutrition-
Society, Coleraine, 16-19 Jul 2007, Cambridge University Press, Cambridge 2008, p. 54-59.
- B. Mitterndorfer, Insulin resistance: sex matters, Curr. Opin. in Clin. Nutr. and Metab. Care, (2005), 8, 367.


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5371495]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5371495]

![]()
![]()
![Image taken from ref.[2]](Thankful.gif)






 Imidazolines, with functional group shown, act as binding sites for essential proteins in the human brain [18] and influence the secretion of insulin at the same time by acting on KATP-channels, the most interesting bit being that regioisomers and enantiomers can have entirely different effects [19].
Imidazolines, with functional group shown, act as binding sites for essential proteins in the human brain [18] and influence the secretion of insulin at the same time by acting on KATP-channels, the most interesting bit being that regioisomers and enantiomers can have entirely different effects [19]. Starting from blood glucose levels upon the fed state, insulin has the main role to increase the rate of glucose oxidation (glycolysis) in the liver and muscle while at the same time it converts glucose concentrations to glycogen (the form in which glucose can be stored in the body)[6]. These processes are supported by an increase in the number of glucose transporters to the cell membrane which have a significant role, as described above, as well as by a variety of phosphorylation and dephosphorylation processes (Scheme 1) for activation and deactivation of enzymes (biological catalysts). In order for an enzyme to be phosphorylated, a PO32- group is donated to it by
Starting from blood glucose levels upon the fed state, insulin has the main role to increase the rate of glucose oxidation (glycolysis) in the liver and muscle while at the same time it converts glucose concentrations to glycogen (the form in which glucose can be stored in the body)[6]. These processes are supported by an increase in the number of glucose transporters to the cell membrane which have a significant role, as described above, as well as by a variety of phosphorylation and dephosphorylation processes (Scheme 1) for activation and deactivation of enzymes (biological catalysts). In order for an enzyme to be phosphorylated, a PO32- group is donated to it by 

![Diabetes cartoon, from Ref.[2].](meds.gif) The main disease which greatly involves insulin is known to be diabetes mellitus. The term 'mellitus' can be translated as honey or sweet-tasting urine and, as Thomas Willis observed in the 17th century, "diabetics p**s a great deal"! Type 1 diabetes is the insulin-dependent diabetes (IDDM) whereas type 2, is the non-insulin dependent (NIDDM). Type-1 diabetes, being the most famous, exists due to damaged β-pancreatic cells and thus their unavailability to excrete insulin in the normal way described above. It can be considered as a genetic problem to be inherited, or may be because of environmental effects [29]. Due to this insulin deficiency, glucose transporters are suppressed and glycogen synthesis is limited, whereas synthesis of glucose and protein degradation are facilitated [30], all being the opposite functions that normal insulin secretion produces. Usually it can be treated by replacing the pancreatic tissue through transplantation [31] since it is greatly damaged, but most people nowadays perform injections or use insulin pumps. On the other hand, in type-2 diabetes the pancreatic β-cells function quite perfectly, sometimes over-secreting insulin, but tissues around remain unaffected by it. It is also related to obesity. Finally gestational diabetes is another type of diabetes appearing only to pregnant women though, as a result of problematic insulin secretion [29].
The main disease which greatly involves insulin is known to be diabetes mellitus. The term 'mellitus' can be translated as honey or sweet-tasting urine and, as Thomas Willis observed in the 17th century, "diabetics p**s a great deal"! Type 1 diabetes is the insulin-dependent diabetes (IDDM) whereas type 2, is the non-insulin dependent (NIDDM). Type-1 diabetes, being the most famous, exists due to damaged β-pancreatic cells and thus their unavailability to excrete insulin in the normal way described above. It can be considered as a genetic problem to be inherited, or may be because of environmental effects [29]. Due to this insulin deficiency, glucose transporters are suppressed and glycogen synthesis is limited, whereas synthesis of glucose and protein degradation are facilitated [30], all being the opposite functions that normal insulin secretion produces. Usually it can be treated by replacing the pancreatic tissue through transplantation [31] since it is greatly damaged, but most people nowadays perform injections or use insulin pumps. On the other hand, in type-2 diabetes the pancreatic β-cells function quite perfectly, sometimes over-secreting insulin, but tissues around remain unaffected by it. It is also related to obesity. Finally gestational diabetes is another type of diabetes appearing only to pregnant women though, as a result of problematic insulin secretion [29].