![]()
![]()
Simon Cotton
Uppingham School, Rutland, UK
![]()
Molecule of the Month September 2001
Also available: HTML version.
![]()
 Indeed, and it smells like it too! It is one of the main odour components of jasmine (right), along with a related molecule called jasmone. Methyl jasmonate (MeJA) makes up some 2-3% of jasmin oil. 15,000 blossoms are needed to give 1.5 g of the oil (10-4 g per blossom). Methyl jasmonate is also a flavour ingredient of semi-black (oolong) and black tea. It is an expensive odour; commercial applications in detergents containing oxidising bleaches use the less reactive reduced dihydro- compounds.
Indeed, and it smells like it too! It is one of the main odour components of jasmine (right), along with a related molecule called jasmone. Methyl jasmonate (MeJA) makes up some 2-3% of jasmin oil. 15,000 blossoms are needed to give 1.5 g of the oil (10-4 g per blossom). Methyl jasmonate is also a flavour ingredient of semi-black (oolong) and black tea. It is an expensive odour; commercial applications in detergents containing oxidising bleaches use the less reactive reduced dihydro- compounds.
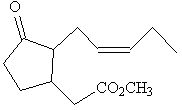 |
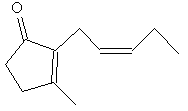 |
| Methyl jasmonate | Jasmone |
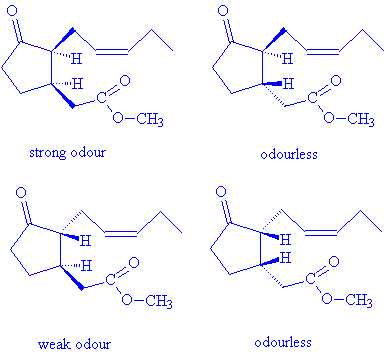
There are two chiral carbon atoms in the methyl jasmonate molecule; each can have either the R- or S- absolute configuration, so that there are four potential isomers. Methyl (+)-epijasmonate, (3R,7S)-(+)-methyl 3-oxo-2-(2-(Z)-pentenyl)cyclopentane-1-acetate, has the strongest odour of the isomers, demonstrating the importance of molecular shape in fitting receptors and activating the sensory response.
But there's much more to methyl jasmonate than a pretty smell.
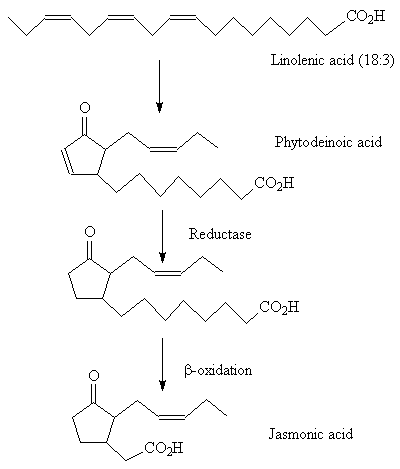
Methyl jasmonate occurs widely in plants. Its biosynthesis starts with linolenic acid and proceeds through a number of stages involving lipoxidation, cyclisation and β-oxidation.
 What does methyl jasmonate do?
What does methyl jasmonate do?Plants that come under attack by insects or damaged mechanically produce higher levels of jasmonic acid and methyl jasmonate, which build up in the damaged parts of the plant. A model has been proposed in which wounding and systemin activate a lipase enzyme in receptor cell membranes resulting in the release of linolenic acid (the precursor of jasmonic acid) and activation of proteinase inhibitor genes. If ingested by the insect, the proteinase inhibitors can interfere with its digestive system and deter the insect from feeding. An 18-amino acid peptide called systemin (Ala-Val-Gln-Ser-Lys-Pro-Pro-Ser-Lys-Arg-Asp-Pro-Pro-Lys-Met-Gln-Thr-Asp) is the polypeptide wound signalling molecule.
Since methyl jasmonate is more volatile than jasmonic acid, it can act as a messenger to neighbouring undamaged plants, telling them that an attack is under way and prompting them to produce defensive chemicals before they are attacked.
Another form of signalling in herbivore-damaged plants can occur when oral secretions of the herbivore contact damaged plant tissue; this prompts the release of volatile organic molecules that serve as attractants for predators of the herbivores, and it seems that methyl jasmonate may be involved in this too.
By no means. Paclitaxel (taxol), obtained from yew trees, is the most important anticancer drug in existence; it is difficult to produce enough paclitaxel to satisfy demand, since it is widely used to treat breast cancer and ovarian cancer in particular. One promising method for its production involves cell cultures from the yew tree. Scientists have discovered that adding methyl jasmonate to the cell cultures greatly increases the amount of paclitaxel produced.
Another promising use for methyl jasmonate lies in prolonging the shelf-life of fresh fruit. It has been shown to reduce chilling injury as well as preventing the growth of mould on strawberries and grapes, as well as stopping bananas from turning brown. The role of methyl jasmonate is again believed to involve the production of defence proteins, which encourage formation of fungicides and antibacterial agents.
![]()
![]()