|
LAURIC ACID(the main constituent of coconut oil)
Paul May
Also available: HTML, and VRML versions.
|
 |
|
LAURIC ACID(the main constituent of coconut oil)
Paul May
Also available: HTML, and VRML versions.
|
 |
![]()
Yes, in fact around 50% of the content of coconut flesh is lauric acid (a.k.a dodecanoic acid), a fatty acid. [1,2]
 Lauric acid
Lauric acid
 Does that mean that eating coconuts is bad for your heart?
Does that mean that eating coconuts is bad for your heart?Yes, and no. Fatty acids are known to increase cholesterol in the bloodstream, which can lead to blocking of the blood-vessels in the heart prodcing heart attacks. And lauric acid is also known to produce the largest increase in total cholesterol of all the fatty acids. But this isn't as bad as it seems, because most of this increase is due to an increase in so-called 'good' cholesterol. Medics have classified cholesterol into two types, HDL and LDL, depending on the density of the lipoproteins. Low-density lipoproteins (LDL) are very bad for you, as they greatly increase the chance of getting arterial blockages, whereas HDL are not such a problem. As a result, medics believe that lauric acid lowers the total:HDL cholesterol ratio more than any other fatty acid, either saturated or unsaturated [3]. So eating cocunuts may actually decrease the risk of getting heart disease [4], although that has yet to be properly proven.
It comprises 6.2% of the total fat in human-breast milk, 2.9% of that in cow's milk and 3.1% goat's milk [2].
Beause it is relatively cheap, has a long shelf-life, is non-toxic and safe to handle, it is often used for the production of soaps and cosmetics. The lauric acid is extracted, then neutralized with NaOH to give sodium laurate, which is a soap. This can be further processed to give sodium laurel sulfate, a detergent found in many household cleaning products such as shampoo and toothpaste.
Lauric acid is also known to the pharmaceutical industry for its excellent antimicrobial properties, and the monoglyceride derivative of lauric acid, monolaurin, is known to have even more potent antimicrobial properties against lipid-coated RNA and DNA viruses, numerous pathogenic gram-positive bacteria, and various pathogenic protozoa.
Because pure lauric acid has a relatively high melting point (43.2°C) it is often used to determine the molar mass of an unknown substance via the freezing-point depression method. By melting it, allowing it to cool, and then measuring the temperature at which a mixture of lauric acid and an unknown substance freezes, the molar mass of the unknown compound may be determined [5].
 What about coconut oil?
What about coconut oil?As well as foodstuffs, coconut oil can be used as a feedstock for biodiesel fuel. Various tropical-island countries are using coconut oil as a fuel source to power trucks, automobiles, and buses, and for electrical generators. Before electrical lighting became commonplace, coconut oil was used for lighting in India and was exported under tha name cochin oil [6]. Coconut oil can be used as a skin moisturizer, as an engine lubricant and a transformer oil, and acids derived from coconut oil can be used as a herbicide [7]. Coconut oil is used by movie theatre-chains to pop popcorn!
Much of it gets converted into monolaurin (a.k.a. glyceryl laurate), which may help protect against bacterial infection [8]. Because of this, monolaurin is commonly used in deodorants. Finally, monolaurin gets converted into the HDA version of cholesterol.
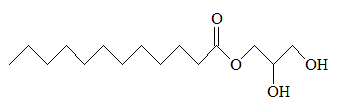 Monolaurin
Monolaurin
![]()
 Some assorted facts about coconuts...
Some assorted facts about coconuts...![]()
1. D.J. Anneken, S. Both, R. Christoph, G. Fieg, U. Steinberner, A. Westfechtel, "Fatty Acids" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a10_245.pub2
2. J. Beare-Rogers, A. Dieffenbacher, J.V. Holm, (2001). "Lexicon of lipid nutrition (IUPAC Technical Report)". Pure and Applied Chemistry 73 685. doi:10.1351/pac200173040685
3. R.P. Mensink, P.L. Zock, A.D.M. Kester, M.B. Katan (2003). Am. J. of Clin. Nutr. 77 1146.
4. M.A. Thijssen R.P. Mensink. (2005). Fatty Acids and Atherosclerotic Risk, in A. von Eckardstein (Ed.) Atherosclerosis: Diet and Drugs. Springer. pp. 171.
5. "Using Freezing Point Depression to find Molecular Weight". University of California, Irvine. 2010-04-12.
[6] G.S. Brady, H.R. Clauser, J.A. Vaccari,(2002). Materials Handbook - An encyclopedia for managers, technical professionals, purchasing and production managers, technicians, and supervisors (15 ed.). McGraw-Hill. pp. 250-251.
[7] T.K. James, A. Rahman, (2005), New Zealand Plant Protection 58 157.
[8] Wikipedia: Coconut oil, lauric acid, monolaurin.
![]()