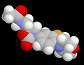
| This is the molecule of linezolid - ZyvoxTM. Chemically, linezolid is (S)-N-[[3-(3-fluoro-4-morpholinylphenyl)-2-oxo-5-oxazolidinyl]methyl] acetamide. The key structural feature of the molecule is the N-aryloxazolidinone moiety, which is indicated in green. Therefore, linezolid and related compounds with antibacterial activities have been known as oxazolidinone antibiotics.

Oxazolidinone antibiotics - historical perspective
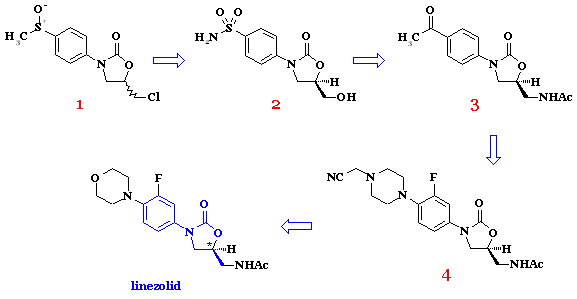
In 1978, oxazolidinones (sulfoxide 1) were first described for their utility for treating plant diseases. Antibacterial properties were discovered six years later, specifically, sulfonamide 2 exhibited modest efficacy against several strains of bacteria [1]. Structural variations of 2 led to DuP-721 (3) and DuP-105 (1987), with greatly improved antibacterial properties relative to their progenitor compounds [2]. It is usually these two compounds, which are referred to as the first true lead compounds in the oxazolidinone family (lead compound - compound that exhibits pharmacological properties which suggest its value as a starting point for drug development). Further structural elaboration led to piperazine derivatives (e.g., 4) [3]. Those compounds were selected for further modification as they combined excellent activity with an acceptable safety profile. Linezolid emerged in 1996 as a result of intensive investigations at Pharmacia [4] and has since then itself been a lead compound [5]. Linezolid was approved by FDA in 2000. The effort and time to accomplish such so small structural modifications may well demonstrate how vast the chemical space is! It is worth emphasizing that the oxazolidinones are the only new class of antibiotic that have been discovered and successfully implemented in the clinic over the past 40 years.

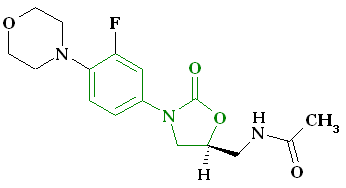
Pharmacophore
Pharmacophore is referred to as spatial arrangement of atoms a molecule must possess in order to exhibit the desired biological effect. This fixed ensemble of steric and electronic properties in the ligand corresponds well to that in its protein receptor. This enables strong interaction and hence the biological effect. Pharmacophore does not represent a real molecule but rather a feature that a molecule carries (phoros). This feature ensures that the molecule possesses a drug's (pharmacon) biological activity. In order for a linezolid-type of drug to be active, it must possess an aryl group on the oxazolidinone ring. Also, stereochemistry at C-5 is critical [6]. Aromatic fluorine substituent improves bioavailability and increases potency, but it is not critical. Likewise, the role of the morpholino group at the para position is to ensure good safety profiles. Oxazolidinone pharmacophore is indicated in the above figure in blue.

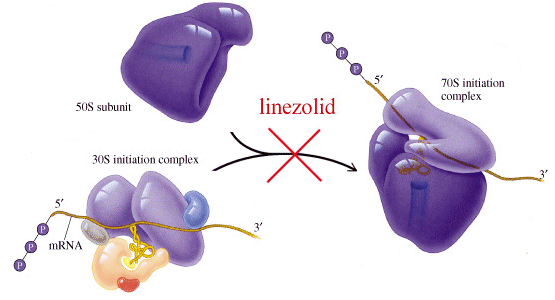
Oxazolidinone antibiotics - mode of action
There is an ongoing need to synthesize new and improved antibiotics since bacteria develop various and (sometimes fascinating) mechanisms of resistance for the existing ones. For years, physicians held hope that vancomycin was the most reliable antibiotic to treat resistant bacteria strains. Isolation of methicillin-resistant Staphylococcus aureus for the first time in 1996 happily coincided with the introduction of linezolid in the same year. Synthetic antibiotics represent human efforts to interfere with the bacterial life cycle, as opposed to Nature's attempts in the form of natural antibiotics. Chemists and molecular biologists can therefore employ tricks which may lead to superior antibiotics and that was the case for linezolid. While most of the widely known antibiotics (macrolides, chloramphenicol) inhibit bacterial protein synthesis at the peptide chain elongation stage, linezolid acts early by potent interaction with 50S ribosomal subunit [7]. In the initiation step of bacterial translation, 50S subunit is associated with fMet-tRNA and a complex composed of 30S ribosomal subunit and mRNA to form the functional initiation complex. Linezolid interacts with the peptidyl-tRNA binding P site at the 50S subunit with micromolar affinity [8] (and it has no affinity to the 30S subunit [9]). This interaction prevents binding of fMet-tRNA to this site during the formation of the initiation complex. In an elegant study, precise binding site has been elucidated by in vivo cross-linking of a photolabile linezolid derivative bound to the 50S subunit [10]. It was found that linezolid interacts specifically with 23S rRNA by a yet unidentified mechanism.

Mechanisms of resistance
Linezolid has been used to treat a number of resistant strains of bacteria (e.g., methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium [11]). However, the emergence of linezolid resistance has been reported, initially in Enterococcus species [12]. More recently, resistant strains of MRSA [13], E. coli [14] and other bacteria [15] have also been identified. Target site modification is virtually the only mechanism of resistance that has thus far been described. Mutations identified in various species are associated with G to U substitution in the peptidyl transferase center of 23S rRNA at position 2576 and result in reduced affinity of linezolid to the 50S subunit [16]. This and other sites of mutations (e.g. T2500A in S. aureus [17]) lie in the proximity of P site, which confirms the mechanism of action of oxazolidinones. In addition, one report describes a non-ribosomal mechanism of resistance in Mycobacterium smegmatis [18]. Ribosomes isolated from these strains behaved essentially like wild-type ribosomes in the presence of drug. It is speculated that the resistance may arise from decreased uptake or increased efflux of the drug. [19]

Metabolism
Linezolid is metabolized via morpholine ring oxidation. In humans, approximately 30% of a linezolid dose is excreted in the urine as the parent drug [19]. Radiolabel studies using [14C]linezolid show that the total recovery of drug-related radioactivity is near quantitative in 48h. In human liver microsomes, linezolid is oxidized to a single metabolite, PNU-143011 [20]. In vitro studies with microsomes suggest that this is a chemical rather than enzymatic reaction. The resulting hemiacetal metabolite (PNU-143011) is unstable and it is either reduced or oxidized enzymatically in in vivo conditions. Oxidation yields PNU-142586, which is the major urinary metabolite (45% of human excreta). Since it is a delta-hydroxyacid, the name lactone pathway has been coined for this route. Similarly, secondary metabolite (PNU-142300; 11%; delta-amino acid) arises from lactam pathway, which is unrelated to the lactone pathway and is presumed to be purely enzymatic. The two major metabolites do not appear to have significant toxicity (and antimicrobial activity), and accordingly linezolid does not need to be altered for patients with renal impairment [21]. The most abundant minor metabolites - PNU-173558, PNU-143131, PNU-142618 - account for approximately 3.3, 1.0 and 1.0% of dose, respectively. Patterns of metabolism are preserved across species [22]. Metabolites in dog and rat exctera are qualitatively comparable to humans. The major difference lies in the relative proportions of the lactone and lactam pathways. In human, the lactone pathway is favored (4:1), the dog metabolizes linezolid by the lactone and lactam pathway about equally, and the rat favors the lactam pathway (1:4). Dogs, rats and humans all excrete a similar amount of intact linezolid. These main metabolic pathways are schematized in the picture below.
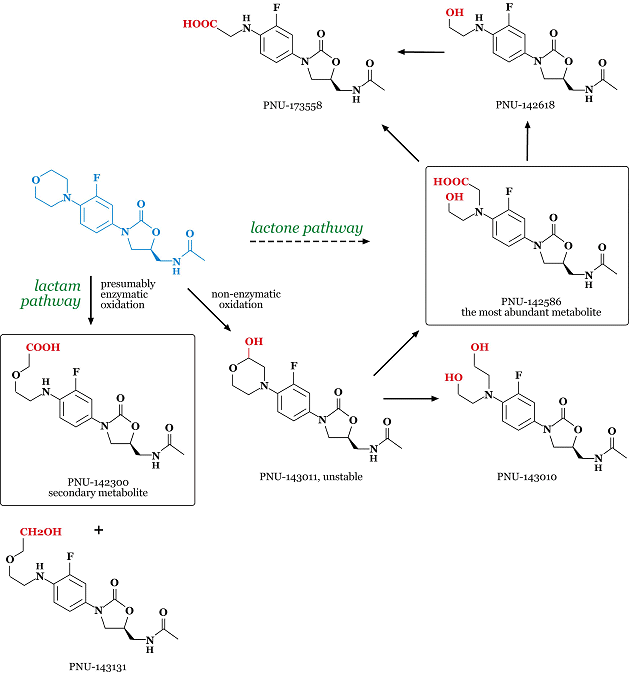

Side effects
In clinical studies on 828 patients, 314 adverse effects were recorded in 223 (27%) of them [23]. The most frequently reported side effects (10%) were gastrointestinal in nature - nausea and, less frequently, diarrhea and vomiting. The other adverse effects included headache, tongue discoloration and oral monilia. More serious side effects resulted in discontinuation of linezolid therapy. Those included thrombocytopenia (condition in which there is an abnormally small number of platelets in the circulating blood) [24], gastrointestinal bleeding and decreased haemoglobin levels. Long-term treatment resulted in fully reversible myelosuppression [25]. Linezolid-induced neuropathy was also reported among patients receiving linezolid for more than 6 months [26]. It is known that linezolid is a weak and nonselective inhibitor of monoamine oxidase [27], yet no cardiovascular events associated with this action were observed. In all cases, no life-threatening adverse events were reported. However, it must be emphasized that long-term side effects may take years to develop.

References


  Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5436826] Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5436826]
 |
|