Vancomycin - a vital antibiotic
[Molecule of the Month - July 1997]
Strains of microorganisms that have multiple resistance to antibiotics are one of the most serious problems encountered by hospitals in Europe and the United States. Vancomycin is sometimes the only available antibiotic left that is effective against such microbes [1, 2]. Vancomycin, the first of a series of chemically related antibiotics, was isolated over 40 years ago in the Eli Lilly Company laboratories in the USA from a Streptomyces species found in soils obtained from Borneo and India. Since then producers have been found in similar locations all over the World. The original producer was given the name Streptomyces orientalis, but in recent changes to bacterial nomenclature it is now referred to as Amycolatopsis orientalis.
Structure of vancomycin
Vancomycin is an glycopeptide antibiotic. Its structure is indicated in the figures below.
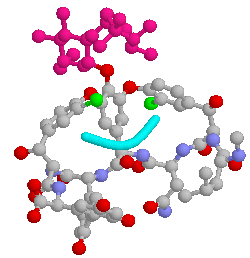
This image was generated using Rasmol. It shows vancomycin in 'ball and stick' conventions, with the backbone of a target molecule highlighted in cyan.
|
Taken from 'Biotechnology of Antibiotics and Other Bioactive Microbial Metabolites' by G. Lancini and Rolando Lorenzetti |
This figure shows the chemical structure of vancomycin, with that of its target D-alanine-D-alanine.
You can download the file of 3D co-ordinates for the atoms in vancomycin. The Protein Data Bank accession number is 1VAN.
Vancomycin action and resistance
The main target of this antibiotic is the D-alanyl-D-alanine terminal dipeptide of peptidoglycan precursors, used by bacteria for constructing their cell walls. This prevents the reaction used to link peptidoglycan precursors together from taking place. Vancomycin binds with the substrate, not the enzyme: this is in contrast to the way penicillin inhibits peptidoglycan synthesis.
With the increased use of vancomycin it is not surprising that resistance to it can now readily be observed especially in Enterococci, a group of bacteria that account for over 10% of all hospital acquired infections in the United States. Generally, resistance to vancomycin is carried by bacterial 'jumping genes'. A well known example of vancomycin resistance is the VanA phenotype, charcaterised by high-level resistance to vancomycin and another glycopeptide antibiotic teicoplanin . VanA phenotype genes are carried on a transposon designated as Tn1546. Tn1546 carries cluster of seven vancomycin-resistance genes, five of which are required for expression of the VanA phenotype, and include theVanA genes [3]. Resistance can be spread by transfer of these genes between organisms. Unbelievably, the European Scientific Committee for Animal Nutrition (SCAN) have approved the continued use of the antibiotic avoparcin, an analogue of vancomycin known to select for resistance genes that confer resistance to vancomycin.
For an indication of how it is used in the clinic Cedars-Sinai Medical Centre has some details of the use of vancomycin. NICL Laboratories has a description of Vancomycin Resistant Enterococci
Approaches to overcoming resistance
The structure of vancomycin (like penicillin) enables some modifications that allow it to retain its antibiotic properties, but overcome many of the resistance mechanisms observed to date. The molecule is a glycopeptide with the sites that bind to the peptidoglycan precursors located in the petide core. Natural producers of the antibiotic add sugars to the hydroxyl group of one of the core amino acid side chains. By changing the nature of these sugar residues [4], semi-synthetic vancomycin analogues have been produced that are active in vivo against many of the most important species of bacteria that are resistant to vancomycin. Recently it has been possible to determine the crystal structure of vancomycin [5] and begin to understand why such structural modifications are effective. The generation of stable vancomycin dimers seems to be involved.
New possibilities for production
Jim McKintyre, working with Dr Alan Bunch and Prof Alan Bull at the University of Kent has shown for the first time that vancomycin can be synthesised in continuous culture [6]. In a subsequent paper by this group, it will be shown that adding biomass recycle to this type of bioreactor enables a significant improvement in vancomycin productivity [7]. This thus allows the application one of the most important investigative tools in microbiology to the manipulation of vancomycin production and possibly the synthesis of more novel antibiotics.
 Back to Molecule of the Month page [DOI:10.6084/m9.figshare.5469580]
Back to Molecule of the Month page [DOI:10.6084/m9.figshare.5469580]