![]()
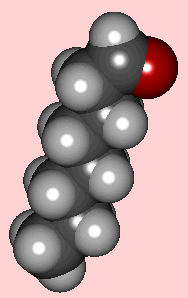
Octanal
The smell of oranges that
birds use as 'perfume'
![]()
Simon Cotton
Uppingham School, Rutland, UK
![]()
Molecule of the Month May 2011
Also available: JSMol version.
![]()

 |
OctanalThe smell of oranges that
|
 |
 What perfume's your bird wearing?
What perfume's your bird wearing?Aldehydes, especially octanal.
No, that's mainly bigger aldehydes, including 2-methylundecanal.
During its breeding season in the Bering Sea off Alaska and the Okhotsk Sea off Siberia, the crested auklet (Aethia cristatella) emits a strong tangerine-like perfume. Pairs 'alloanoint', rubbing their bill, breast, head, and neck over the wick feathers of their partner, spreading scent molecules over the head, neck, and face areas where the birds cannot self-preen.

|
|
This substance is a mixture of even-carbon aldehydes especially hexanal, octanal and decanal, as well as the unsaturated aldehydes (Z)-4 decenal, (Z)-4-dodecenal and (Z)-6-dodecenal. Analysis shows that octanal is present in more than twice the amount of the next most abundant aldehyde.
In contrast, the whiskered auklet (Aethia pygmaea) uses an odorant that is a mixture of mainly the odd-numbered aldehydes heptanal and nonanal.
It is believed that the aldehydes may be repellents for pests such as arthropods and ectoparasites. It has been shown that the auklet's emissions repel female Aedes aegypti mosquitoes; there also appears to be a correlation between low chemical emission rate by the bird and high infestation by ticks. Along with heptanal, nonanal and decanal, octanal is one of the molecules emitted from human skin that draws a response from female Aedes aegypti mosquitoes and recent research indicates that octanal, nonanal and decanal are natural repellents for mosquitoes. Orange oil is reportedly very effective against the Formosa termite, Coptotermes formosanus Shiraki, but this effect is believed to be due to limonene.
Indeed. Octanal is found both in orange oil and orange juice, though the amount varies from one variety to another, and fresh juice is unstable. The main component of orange oil is (R)-(+)-limonene, which has a very weak smell - if you buy limonene from commercial sources, it contains significant amounts of smelly aldehydes and other impurities. The fresh smell of fresh orange juice and of oil squeezed from the peel is attributed largely to the C8-C11 aldehydes octanal to undecanal, as well as to unsaturated aldehydes like neral and the sinesals. Esters including ethyl butanoate, ethyl octanoate and ethyl decanoate are also important.
It is a colourless strong-smelling liquid at room temperature, with a boiling point of 163.4°C. It's used industrially to supply an orange "note", especially to perfumes and food preparations.
At the moment, we still do not know precisely how odorant molecules are detected in our noses, but it is known that humans have around 350 different smell receptors, and that each receptor responds to some molecules, but not others. A molecule will usually produce a response from several receptors, and the "smell" is the brain's response to the pattern of signals arriving from all the receptors activated by that molecule. An MRI study was carried out on the mouse olfactory bulb when the mouse was exposed separately to each one of the C4-C8 aldehydes; each one caused different globular activity patterns, corresponding to different "smells".
The mouse I7 odorant receptor differs from the rat I7 receptor by a single valine-to-isoleucine substitution; this gives its strongest response with heptanal. The rat I7 odorant receptor (OR-17) gives a strong response to straight chain C7-C10 aldehydes, most strongly to octanal, but not to aldehydes with less than 7 or more than 10 carbons. Short-chain aldehydes appear to bind to the receptor but not to activate it; aldehydes above C11 are too big to fit the binding pocket. Some unsaturated aldehydes activate it too, but few other odorants - no ketones and esters for example, and not alcohols like octan-1-ol.

"'Ere, you looking at my bird?"
![]()
Chapman and Hall Combined Chemical Dictionary compound code number CWB57-S (props, bibliography)
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255422]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5255422]