![]()
Octane
The molecule in petroleum
used for car engines
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month February 2019
Also available: HTML version.
![]()

 |
OctaneThe molecule in petroleum
|
 |

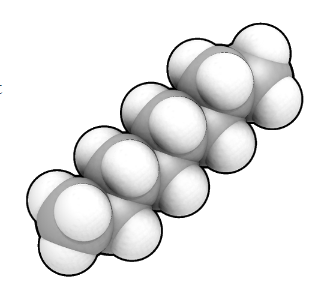
Yes, it's a long, straight molecule of octane.
 Is that anything to do with the 95 and 97 or 98 on petrol pumps?
Is that anything to do with the 95 and 97 or 98 on petrol pumps?Yes, although there is more to it than octane ratings.
Well, octane is a hydrocarbon, a molecule made up of just carbon and hydrogen atoms bonded together. It has 8 carbon atoms; that is why the name begins with oct-.
Octane belongs to a family of molecules called alkanes. These are hydrocarbon molecules with just single, two-electron, bonds connecting the atoms. There is a whole series of them with increasing numbers of carbon atoms, starting from methane (1 carbon), ethane (2 carbons), propane (3), butane (4), etc. If there was a double bond in the molecule it would be an ‘alkene’ and the name would end in –ene (ethene, propene, etc.).
Where does octane come from?
How do they get octane from the crude oil?They refine the petroleum. This involves boiling the crude oil and passing the vapour up a ‘fractionating tower’ that is hot at the bottom and cold at the top (see diagram, right). Because the different-sized molecules have different boiling points, they condense out from the vapour at different points. There are trays at different levels of the tower that collect ‘fractions’, molecules with similar boiling points. Why do molecules have different boiling points?When you boil a liquid and convert it to a gas, the molecules are separated from each other and move much further apart. For this to happen, you have to overcome the attractive forces between the molecules. These ‘intermolecular forces’ are weaker than the chemical bonding forces that hold molecules together; think of them as ‘Molecular Velcro’ if you like. The bigger the molecule, the stronger the intermolecular forces. So molecules of roughly similar size collect in the same ‘fraction’, though a fraction still contains lots of different molecules. |
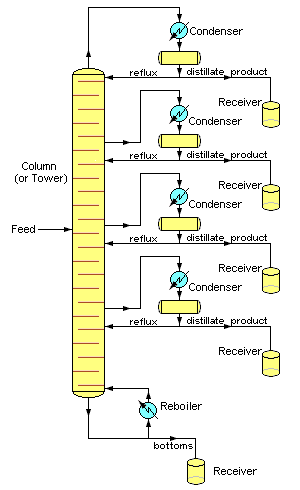 Simplified chemical engineering schematic diagram of a Continuous Fractional Distillation tower separating one feed mixture stream into four distillates and one bottom fractions [Photo: H Padleckas CC BY-SA 3.0] |
Further careful fractional distillations are needed. You can also get octane by the process known as cracking.
Maybe, but cracking involves passing the vapour of big molecules over a hot catalyst, which splits them into smaller molecules. An example of a product produced by cracking is an alkene such as ethene (MOTM December 2006); these reactive molecules are very useful to the chemical industry.
In essence, cracking means reactions like:
Big alkane  Smaller alkane + alkene
Smaller alkane + alkene
So you can get octane (amongst other molecules) by cracking decane, for example.
C10H22  C8H18 + C2H4
C8H18 + C2H4
You can also make other alkanes by ‘isomerisation’ reactions using appropriate catalysts.

Picture of a cracker in Baton Rouge (straight out of the Johnny Cash songbook).
[Photo: WClarke - Own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=57197981]
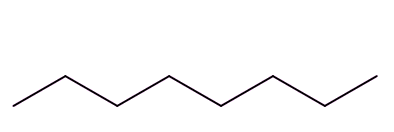
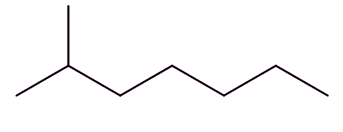
Well, isomers are compounds with the same molecular formula but different structures. For example, octane has lots of isomers, like these three, all variants on C8H18:
 |
 |
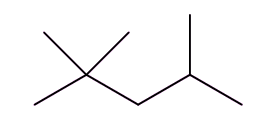 |
| Octane | 2-Methylheptane | 2,2,4-Trimethylpentane |
So, the term isomerisation means converting one isomer into another one. In the chemical industry this usually means converting a straight-chain octane into an isomer with more branches; these are valuable on account of their higher 'octane ratings'.
First, you have to understand the engines in petrol-fuelled cars. Petrol engines are driven by the combustion of a mixture of a gaseous mixture of air and hydrocarbon fuel. This mixture is injected into the cylinder as the piston is on its downstroke, then gets compressed as the piston moves up. At a particular point, a sparking plug fires a spark that ignites the mixture, forming a very hot mixture of carbon dioxide and steam, creating the power that forces the piston down, driving the engine and powering the wheels. As time went on, manufacturers developed engines where the fuel-air mixture was compressed more before it was ignited – with higher ‘compression ratios’. This gave more power, but it was also likely that the fuel-air mixture would pre-ignite, before it was sparked. This caused the engine to misfire, known as ‘knocking’ which would result in less power, not more.
So how did they solve that problem?This was the work of an American chemist called Russell Marker (photo, right). He was employed by the Ethyl Corporation, who played a leading role in developing tetraethyllead (MOTM Jan 2001) as an antiknock agent. Marker examined a number of alkanes that were found in the ‘petrol’ fraction from refining, looking at their ‘knocking’ characteristics. He started with heptane, a seven-carbon molecule that was very similar to octane. Both heptane and octane are known as ‘straight-chain’ alkanes; in this context ‘straight’ means that the chain of carbon atoms in the molecule is unbranched, as the chains are anything but straight, with a zig-zag chain of carbon atoms. Heptane caused really bad knocking, so next he tried 2,2,4-trimethylpentane, traditionally known as isooctane; it also has the formula C8H18 so is an isomer of octane. This has a branched chain containing eight carbon atoms, but does not cause any knocking. Like heptane, octane, with its ‘straight’ chain of eight carbons, was bad too. |
 This photograph of Russell Marker doing some gardening appeared on the August 1951 cover of Tiempo magazine. [Photo: Penn State Room, Penn State Libraries.] |
Mixtures were made with varying amounts of heptane and isooctane, so that their knocking characteristics could be studied. A fuel described as 95 octane has the same knocking characteristics as a mixture containing 95% isooctane and 5% heptane. The higher the octane rating, the more the gas-air mixture can be compressed before it is ignited, and the more power you get when it burns.
Yes, it means with the right fuel mixture, engines runs more smoothly, efficiently, and quietly. And until the 1990s, it was improved still further by the addition of tetraethyllead as an additional anti-knock agent. Nowadays, however, cars use 'lead-free' fuels due to issues with lead being found in the environment and the fact that it damages the catalytic converters that are fitted to modern cars (see MOTM Jan 2001).
Cost. It costs quite a lot of money to separate the isooctane from the other less desirable alkanes, so a mixture is used to keep costs down. But 100-octane fuel is used for some aircraft engines, and if you go back to the last century, high-octane fuel helped shape history.
Back in 1927-1931, British seaplane aircraft won the Schneider trophy (a speed race for airplanes) for Britain three times, with the help of high-octane fuels developed by Air Commodore Rod Banks and his colleagues. The designer R. J. Mitchell later developed these seaplanes into the Supermarine Spitfire fighter aircraft. 100-octane fuel originating in the refineries in Aruba, off the Venezuelan coast, found its way into squadron use with the RAF’s Hurricanes and Spitfires in 1939-40, just in time for the Battle of Britain. At the same time, the Luftwaffe’s Me(Bf)109 aircraft were also running on 100-octane fuel, so without it the RAF’s fighters would have been at a considerable disadvantage, and the Battle of Britain may have had a different ending.
 |
 |
| A Supermarine Racing Seaplane | A Supermarine Spitfire [Photo: Alan Wilson from Stilton, Peterborough, Cambs, UK, CC BY-SA 2.0 via Wikimedia Commons] |
Well, he became one of the few university professors not to have a PhD. Just over 10 years after his work on octane ratings, he found a way of degrading diosgenin, found in Mexican yams, into progesterone, which became known as the Marker degradation, an example of semisynthesis at work. This ultimately led to oral contraceptives. People have won Nobel prizes for less valuable work.
 How do you make alkanes in the lab?
How do you make alkanes in the lab?These days no one takes the trouble, as they can be bought cheaply from chemical suppliers.
Several methods are due to famous chemists of the past. Herman Kolbe (1818-1884) found that alkanes can be made by electrolysis of salts of carboxylic acids. So using sodium pentanoate results in octane (and carbon dioxide) at the anode – and sodium hydroxide and hydrogen at the cathode. Two butyl groups get coupled together to make the octane molecule in this reaction.
Overall: 2 C4H9COONa + 2 H2O 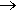 C8H18 + 2 CO2 + 2 NaOH + H2
C8H18 + 2 CO2 + 2 NaOH + H2
In a different type of ‘coupling reaction’, due to Adolphe Wurtz (1817-1884), 1-iodobutane reacts with sodium metal.
2 C4H9I + 2 Na 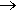 C8H18 + 2 NaI
C8H18 + 2 NaI
Victor Grignard (1871-1935) devised the synthesis of ‘Grignard reagents’, by reaction of clean magnesium metal with halogenoalkanes in an ethereal solvent, so 1-iodooctane reacts with magnesium forming C8H17MgI; if this is hydrolysed, octane is the product.
C8H17I + Mg 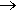 C8H17MgI
C8H17MgI
C8H17MgI + H2O 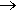 C8H18 + Mg(OH)I
C8H18 + Mg(OH)I
But all these reactions are more expensive (and time consuming) than buying ready-made octane!
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.6139076]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.6139076]