![]()
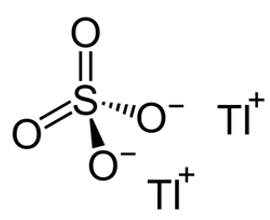
Thallium Sulfate
Rat poison used for Murder
![]()
Mike Thompson, Matty Coe and Thomas Sloan
Rugby School, UK
![]()
Molecule of the Month July 2018
![]()

The green flame test for thallium.
 |
Thallium SulfateRat poison used for Murder
Mike Thompson, Matty Coe and Thomas Sloan
Molecule of the Month July 2018
|
 The green flame test for thallium. |
Well, yes, but it’s a lot more interesting than that. Thallium sulfate is an ionic compound with a giant lattice structure. Sulfates are typically made from the reactions of metals with sulfuric acid (MOTM May 2008). The name thallium, like many other elements, comes from a Greek word, Thallos, which means ‘green shoot/twig’, because thallium produces a green flame when heated in a flame test (see picture, above right), due to the single green spectral line in its emission spectrum.
Thallium’s electron configuration is [Xe] 4f14 5d10 6s2 6p1, which means it readily loses one electron or 3 electrons when ionised. The thallium in thallium sulfate is found in the +1 oxidation state, so there are two Tl+ ions for every sulfate ion, giving it an overall formula of Tl2SO4 (so a more accurate name would be thallium(I) sulfate). Thallium also forms a +3 oxidation state like the other elements in Group 13 (boron, aluminium, gallium and indium). The ‘inert pair’ effect is the reason why thallium prefers the +1 oxidation state. This tendency towards univalency is due to the reluctance of its 6s2 electrons to be lost in reactions.
Thallium is classified as a heavy metal…
No, like lead and mercury. Thallium has a density of 11.8 g/cm3 which makes it one of the densest metals known, even denser than the more familiar heavy metal, lead, with its density of 11.35 g/cm3.
 |
 |
| Thallium sulfate powder, a heavy-metal compound. | Lemmy Kilmister 28/12/15 RIP – a heavy-metal legend. |
 Indeed. Thallium and its compounds are most widely known for their high toxicity and thallium compounds have regularly made the news around the world by being used in numerous homicide (murder) cases. As a white solid, thallium sulfate has a very similar appearance to granulated sugar, although apparently it is tasteless - and we are happy to take others’ word for that! To give an idea of the toxicity of thallium sulfate, a single teaspoon of Tl2SO4 (about 34 g) would be capable of killing 34 people - reason enough for chemists to be banned from eating or drinking in their laboratories. Thallium sulfate has a solubility of 4.87 g in 100 mL of water at 20ºC. So in a mug of tea with about 200 mL water at around 60ºC quite a lot of thallium sulfate (10 times the lethal dose, in fact) would dissolve unnoticed. Fortunately, this substance is difficult to get hold of nowadays.
Indeed. Thallium and its compounds are most widely known for their high toxicity and thallium compounds have regularly made the news around the world by being used in numerous homicide (murder) cases. As a white solid, thallium sulfate has a very similar appearance to granulated sugar, although apparently it is tasteless - and we are happy to take others’ word for that! To give an idea of the toxicity of thallium sulfate, a single teaspoon of Tl2SO4 (about 34 g) would be capable of killing 34 people - reason enough for chemists to be banned from eating or drinking in their laboratories. Thallium sulfate has a solubility of 4.87 g in 100 mL of water at 20ºC. So in a mug of tea with about 200 mL water at around 60ºC quite a lot of thallium sulfate (10 times the lethal dose, in fact) would dissolve unnoticed. Fortunately, this substance is difficult to get hold of nowadays.
 So has it been used for murder?
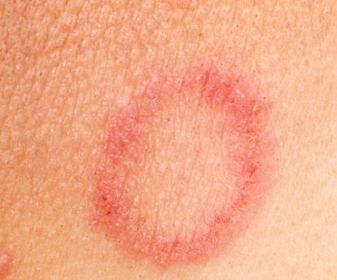
So has it been used for murder?Of course, but we’ll come on to that later. From the 1700s to the 1900s it was actually used as a rather poor medical treatment for ringworm – a fungal infection of the skin which results in an itchy, round rash (see picture, left). Ringworm is transmitted by physical contact with an infected individual and is said to affect 20% of the population at any given time. Thallium sulfate often proved too toxic for the patient, but at least it did kill the ringworm before it killed the patient!
In the 1920s thallium sulfate found widespread use as a pesticide. But this was not without its controversies; in the South American country of Guyana, more than 40 people died from its (mis)use. The reason was that the pesticide, which was left lying around in an attempt to kill rats, where it was absorbed by plants, and then entered the food chain, increasing in concentration (‘bioaccumulation’, see MOTM November 2014 about triclosan), until it was eventually ingested by humans in meat or vegetable foods. It was also found that thallium sulfate had the unfortunate side effect of inhibiting the growth of plants by stopping germination, which reduced crop yields.
 And in Israel its use as a rodenticide is blamed for wiping out the brown fish owl (photo, right) – a local bird of prey that used to eat fish. The thallium compound had seeped into the rivers and lakes and poisoned the fish, which were then eaten by the owls.
And in Israel its use as a rodenticide is blamed for wiping out the brown fish owl (photo, right) – a local bird of prey that used to eat fish. The thallium compound had seeped into the rivers and lakes and poisoned the fish, which were then eaten by the owls.
Not immediately… despite thallium sulfate’s known toxicity its use was not discontinued in Guyana until 1987. Another problem is that the only way to identify patients with thallium poisoning was through blood and urine testing, and this was done only in Guyana’s Hospital in its capital Georgetown. Of the 2,400 people who had either their blood or urine tested, a shocking 77% tested positive for thallium poisoning, showing just how widespread the thallium poisoning had become in the environment.
Thallium sulfate poisoning, much like the deceptive appearance of the compound itself, has initial symptoms typical of a winter virus. This generally means that poisoning is mistaken for a common cold or flu. The symptoms are generally seen in the hours immediately after exposure and can include severe stomach aches as well as diarrhoea. 2-5 days after exposure to thallium the body’s response takes a more neurological turn with numbness and tingling, felt especially on the palms and soles of the feet. This is followed in the weeks after exposure with the growth of a dark pigment at the root of hair leading to baldness. There is also an increase in neurological symptoms such as comas and blindness. These symptoms can sometimes also appear in the days immediately after exposure dependent on the quantity ingested. The compound also affects the heart; initially increasing heart rate and later possibly causing circulatory disturbances that can lead to death. The time that the compound will take to kill you depends on many factors and ranges from as little as 6 days or up to 8 weeks.
Tl+ behaves chemically in a very similar manner to Na+ and K+ ions, and when present in the body it can replace these ions within cells. Since these ions are essential for nerve function, it is therefore no surprise that victims of thallium poisoning often suffer neurological symptoms. The presence of thallium impairs the ability of your cells to produce nerve impulses, and will likely kill you through either respiratory paralysis or circulatory disruption. And it takes very little to be fatal; the lethal dose of thallium sulfate for an adult is thought to range from 500 mg to 1 g.
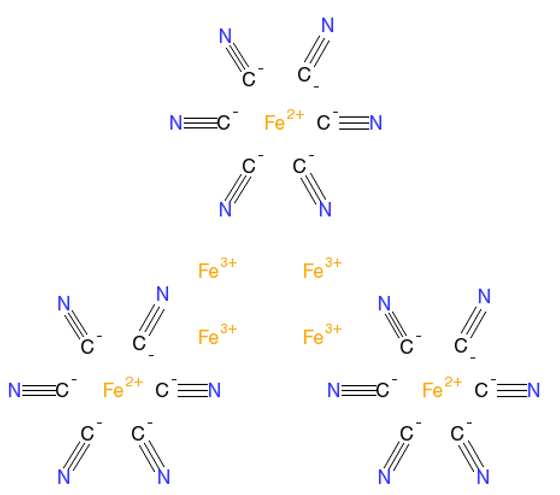
Yes, the antidote, rather surprisingly, is to take tablets of a cyanide-based molecule called Prussian Blue Fe7(CN)18 (see the MOTM for Nov 2011), three times a day for 2 weeks. Prussian Blue is more commonly known as a pigment with a dark blue colour and was the first modern synthetic pigment. It is insoluble in water, but can still be used in paint as it has a very fine colloidal dispersion.
 |
 |
| Prussian Blue powder used as a painting pigment | Vincent van Gogh’s Starry Night which used Prussian Blue. |
 How does it work?
How does it work?Prussian blue has an unusual structure (shown on the right) in that it contains both Fe2+ and Fe3+ ions in the same lattice (for more details see here). It is an ‘ion exchange material’, which means it can swap its central Fe2+ ion for another suitable ion in its surroundings, in this case Tl+. So the Prussian Blue acts as a sponge, soaking up the poisonous Tl+ ions in the body and replacing them with harmless Fe2+ ions. The new complex containing Tl+ renders the metal unavailable for absorption in the stomach and it can safely pass through the intestines and then be excreted.
Sure – in fact thallium has been called ‘Inheritance powder’ and the ‘poisoner's poison’ because thallium is colourless, odourless and tasteless. Its symptoms are often similar to those of a host of other illnesses and conditions, including innocuous ones such as colds or flus, so the victim often doesn’t realise they’ve been poisoned. And its symptoms are so slow-acting they allow the poisoner to be long gone before the victim falls sick.
One of the first notable cases was that of Graham Young, who was born in 1947 in Neasden, London. From an early age, Young was obsessed with poisons. This led him to begin testing different toxic substances on his relatives when he was only 14.
Suspicion was aroused when Young’s family became violently ill, with his sister once hallucinating on the train to work. After she was taken to hospital it was concluded that she had been exposed to he plant Deadly Nightshade (atropa belladonna), but the source was not known.
Eventually his ‘experiments’ killed his stepmother Molly. To destroy evidence of his murder he convinced his father to have her cremated, so that her body couldn’t be tested for the presence of his poisonous chemicals. Next came another mysterious illness of his sister, which led his aunt to become suspicious. Knowing of his love of poisons she suggested taking Young to a psychiatrist. Soon after this consultation he was in the hands of the police.
After being found to possess psychopathic tendencies by pre-trial psychiatrists, he was sent to Broadmoor Hospital, which a high security prison/hospital in Crowthorne in Berkshire. He was their youngest inmate since 1885.
 |
 |
| Graham Young at his first trial aged 15 | Young at his second trial aged 25 |
Young was later released from Broadmoor in 1971 because authorities thought that they had ‘cured’ him of psychopathy. He then went to work as a laboratory technician at a chemical factory in Bovingdon, near Hemel Hempstead. Here he is thought to have been the cause of the “Bovingdon Bug” which affected more than 70 people and killed three. The cause of the ‘bug’ turned out to be Young who was poisoning his co-workers’ tea with thallium sulfate.
 In Australia in the 1950s there were several thallium poisonings, so much so that they became known as Australia's "Thallium Craze". This is partly because at that time thallium sulfate was readily available over the counter as a rat poison.
In Australia in the 1950s there were several thallium poisonings, so much so that they became known as Australia's "Thallium Craze". This is partly because at that time thallium sulfate was readily available over the counter as a rat poison.
In one case,Yvonne Fletcher from Sydney killed two husbands using thallium. She nearly got away with the first murder, but suspicions were raised after it became obvious to friends that her second husband was dying and exhibiting the same symptoms that had killed her first husband. A police investigation found clear evidence of thallium in the remains of the first husband’s body, and this led to Yvonne’s being convicted for murder.
Soon after, grandmother Ruby Norton was tried, but later acquitted, for the murder of her daughter's fiancé Allen Williams, who had died of thallium poisoning. In a sensational trial in 1952, Veronica Monty was tried for the attempted murder using thallium of her son-in-law, well-known Australian rugby-league player Bob Lulham. The trial revealed that Lulham and Monty had had an "intimate relationship" while Lulham's wife was at Sunday mass. Neverthless, Monty was found not guilty, but she later killed herself, ironically using thallium, in 1955.
In 1953 Sydney woman Beryl Hague was tried for "maliciously administering thallium and endangering her husband's life". She confessed to buying thallium sulfate rat poison (under the brand name of ‘Thall rat’) and putting it in her husband's tea. She said she wanted to "give him a headache to repay the many headaches he had given me" in past domestic violence.
And in the same year, Caroline Gills poisoned three members of her family and a friend. Once again the poison was difficult to detect because the thallium sulfate had been added to tea. She spent the rest of her life in jail, where the inmates dubbed her "Aunt Thally” (see newspaper extracts, right).
 What about outside Australia?
What about outside Australia?The leader of Cameroon, Félix-Roland Moumié (photo, right), was assassinated with thallium in Geneva in 1960 by the French secret service (SDECE). And in Kiev in 1987, Tamara Ivanutina and several members of her family were found guilty of 40 cases of poisoning (13 of them fatal) with Clerici solution (a dense thallium-based solution used to test minerals). In 1988, in Florida, members of the Carr family fell ill from thallium poisoning. The mother died slowly and painfully, while her son and stepson were critically ill but eventually recovered. Their neighbour, George J. Trepal, who was a chemist, was convicted of murder and attempted murder, and was sentenced to death. In this case, he had placed the thallium into bottles of Coca-Cola! There are many other cases, but one of the strangest was in 2004, when 25 Russian soldiers earned Honorable Mentions in the Darwin Awards. They all became ill from thallium exposure after finding white powder in a rubbish dump on their base in the far east of Russia. Despite this being an unidentified white powder from a military dump, the soldiers added it to tobacco, and used it as a substitute for talcum powder on their feet!
Crime writers are always looking for ways for their characters to commit crimes. The world-renowned whodunnit author Agatha Christie knew about the symptoms of thallium poisoning (hair loss) when she wrote her book The Pale Horse in 1961. In fact, this novel is unusual in that it probably saved at least two lives after readers recognised the symptoms of thallium poisoning described in the book. In contrast, the same book was found amongst the belngings of two notorious poisoners (George Trepal and his wife Dr Diana Carr), with the implication being that they got their nefarious ideas from the book. Another author, Ngaio Marsh, also gave thallium a mention in the form of thallium acetate in her book Final Curtain published in 1947.
 |
 |
 |
 |
 |
 |
| Books | Movies | and TV | |||
Thallium poisoning has also appeared in numerous movies (such as Big Nothing (2006), The Young Poisoner's Handbook (1955), Edge of Darkness (2010), and the James Bond movie Spectre (2015)) and TV shows (House, NCIS, CSI:NY, and Royal Pains, to name but a few).
 Are there any legitimate uses for thallium sulfate?
Are there any legitimate uses for thallium sulfate?Perhaps the only ethical use of thallium sulfate today is for scientific research. It is currently being used as a source of Tl+ to make thallium sulfide (Tl2S) by the reduction of thallium sulfate.
Tl2SO4 + 4C  Tl2S + 4CO
Tl2S + 4CO
Thallium sulfide has many interesting uses such as in early cinematic projectors which enabled the synchronization between sound and picture (as shown in the photo, right), as well as its use in the development of long-wave infrared detectors in WW2.
![]()
Wikipedia: Thallium sulfate; Thallium poisoning; Prussian Blue; Prussian Blue antidote; Clerici solution; Félix-Roland-Moumié; Thallium sulfide.
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5972410]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.5972410]