![]()
Wilkinson's catalyst
Chlorotris(triphenylphosphine)rhodium(I), [RhCl(PPh3)3]
The famous inorganic catalyst
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month July 2013
Also available: HTML version.
![]()
|
Wilkinson's catalystChlorotris(triphenylphosphine)rhodium(I), [RhCl(PPh3)3]The famous inorganic catalyst
Simon Cotton
Molecule of the Month July 2013
|
|
 It was discovered accidentally. Fred Jardine, a PhD student working for Geoffrey Wilkinson (photo, right), was trying to make [RhCl3(PPh3)3], from the reaction of hydrated rhodium trichloride and excess triphenylphosphine in boiling ethanol. Most of the time he succeeded in making [RhCl(PPh3)3], as usually the excess of phosphine acts as a reducing agent.
It was discovered accidentally. Fred Jardine, a PhD student working for Geoffrey Wilkinson (photo, right), was trying to make [RhCl3(PPh3)3], from the reaction of hydrated rhodium trichloride and excess triphenylphosphine in boiling ethanol. Most of the time he succeeded in making [RhCl(PPh3)3], as usually the excess of phosphine acts as a reducing agent.
[RhCl3(H2O)3] + 4 PPh3 ![]() [RhCl(PPh3)3] + Ph3PO + 2 HCl + 2 H2O
[RhCl(PPh3)3] + Ph3PO + 2 HCl + 2 H2O
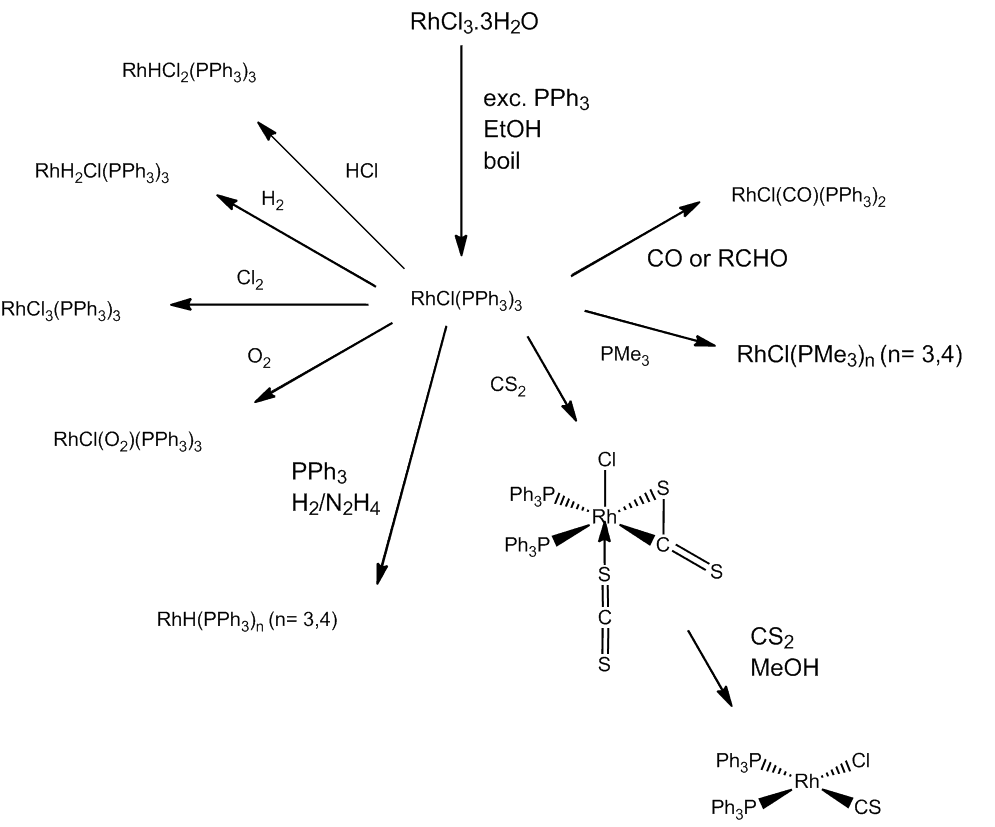
Not at all. Sterically uncongested trialkyl phosphine ligands, give both fac- and mer- [RhCl3(PR3)3] (PR3 e.g. PMe3), whilst bulkier trialkyl phosphines (e.g. PBu3) give just mer-[RhCl3(PR3)3]. In another redox reaction, very bulky phosphines react in the cold to give Rh(II) complexes, trans-[RhCl2(PR3)2] (PR3 = P(o-tolyl)3, PCy3, PBut2R (R = Me, Et, Pr)). At high temperatures bulky trialkylphosphines can give hydrides such as [RhHCl2(PBut2Pr)2] and [RhH2Cl(PBut3)2].
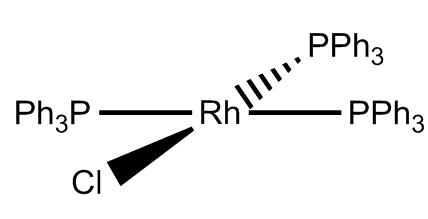
Burgundy coloured crystals, though it is sometimes obtained as orange crystals of another polymorph - which has the same molecular structure.

Left: Spacefill structure of Wilkinson's catalyst. Right: Photo of the burgundy crystals.
Although [RhCl3(PPh3)3] catalyses the reaction of hex-1-ene with H2 and CO, forming heptanal at 55°C, [RhCl(PPh3)3] - which is easier to make - displays much more important properties. It is a very active catalyst for the rapid homogenous hydrogenation (i.e. in solution) of certain unsaturated compounds. It was the first such catalyst to work at comparable rates to the best heterogeneous (i.e. solid) catalyst, working rapidly at 25°C and 1 atm pressure of hydrogen gas. Terminal alkenes are hydrogenated much more rapidly than branched or internal alkenes, whilst reaction with terminal alkynes is much faster than with terminal alkenes. The stereochemistry of addition is cis-
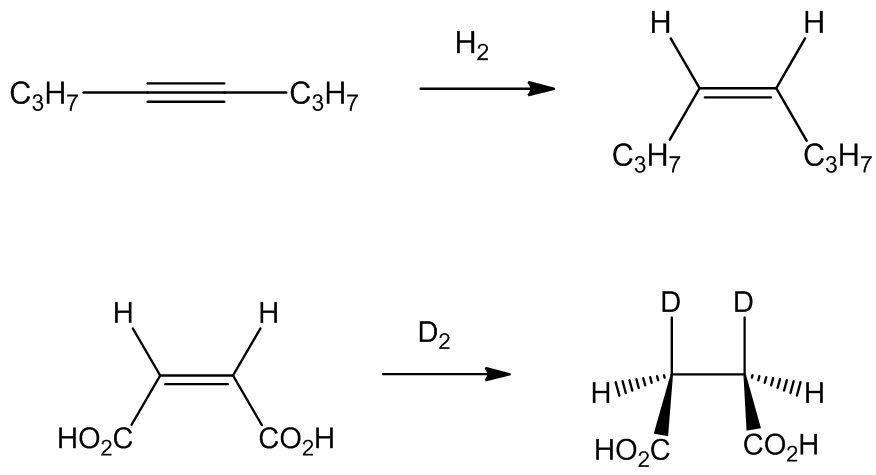
No, [RhCl(PPh3)3] does not reduce benzene rings, nor carbonyl or carboxyl groupings, nor nitrile or nitro (and as already noted, more freely accessible double bonds are reduced preferentially).
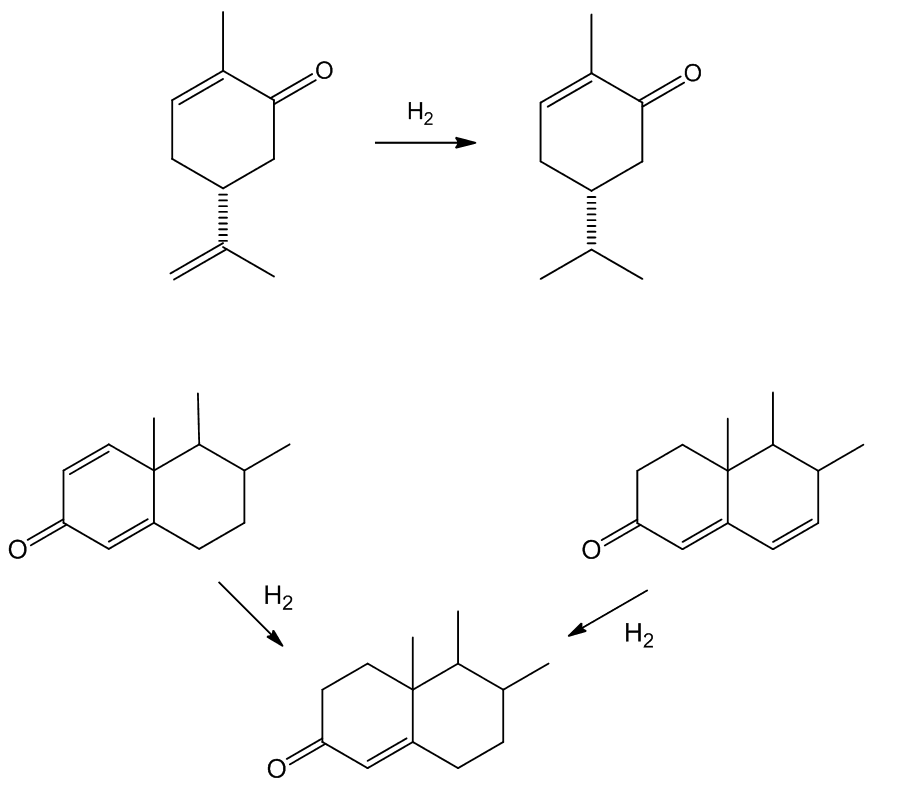
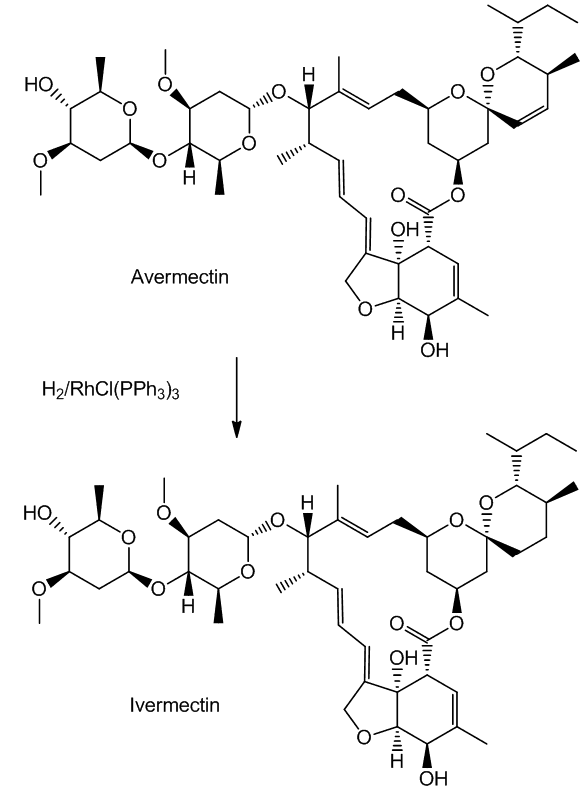
Such selectivity found an important application in the synthesis of Ivermectin (MectizanTM, see MOTM for Oct 2015). Avermectin is a naturally-occurring molecule with anthelmintic and insecticidal properties; selectively reducing one double bond using Wilkinson's catalyst afforded Ivermectin. The resultant small change in molecular shape makes Ivermectin a much more effective drug to combat onchocerciasis (river blindness), a disease which affects many millions of people, mainly in poor African communities.
Polar functions like C=O are usually reduced by ionic groups, like H-. These catalytic reactions of [RhCl(PPh3)3] involve addition of molecular H2 from a covalent dihydride complex to non-polar multiple bonds.
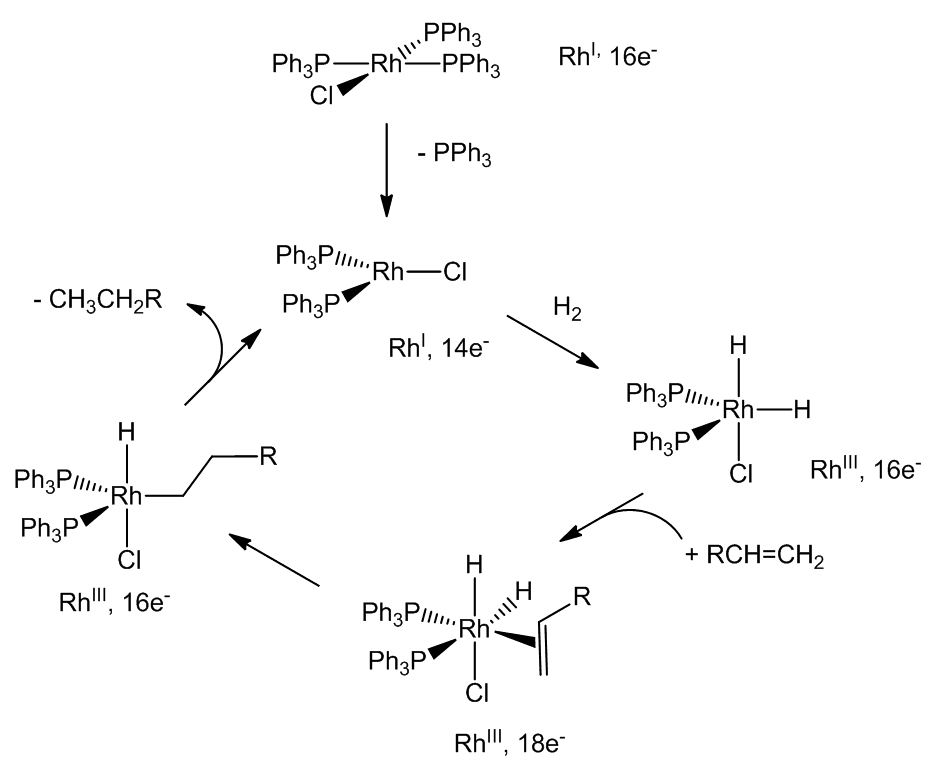
When it is dissolved in a solvent like benzene-ethanol, one of the phosphine molecules can be replaced by a weakly-bound solvent molecule, giving what is effectively a three-coordinate rhodium complex. Under an atmosphere of hydrogen gas, the resulting complex adds a hydrogen molecule, breaking the H-H bond forming a five-coordinate dihydride complex of rhodium. The dihydride is a Rh(III) species, so this is an oxidative-addition reaction, during which the colour of the solution changes from red to yellow. [RhH2Cl(PPh3)2] is still a 16-electron species and also has a vacant coordination site, so it can add an alkene molecule, forming a six-coordinate 18-electron complex. Next, there is a rearrangement with the coordinated alkene being inserted into a rhodium-hydrogen bond to form an alkyl complex - alternatively, think of it as a hydride transfer to the coordinated alkene. This step is rapidly followed by the transfer of the other hydrogen from rhodium to the alkyl group. This generates an alkane, which is immediately lost in a reductive-elimination step, so that the catalytic cycle - shown below in simplified form - can begin again.
Many of its reactions involve addition, often with a change in oxidation state. It reacts readily with CO, forming trans-[RhCl(CO)(PPh3)2], the rhodium analogue of Vaska's compound (MOTM Feb 2013). This is also formed by rapid decarbonylation reactions of aldehydes.
[RhCl(PPh3)3] + CO ![]() [RhCl(CO)(PPh3)2] + PPh3
[RhCl(CO)(PPh3)2] + PPh3
[RhCl(PPh3)3] + C6H5CHO ![]() [RhCl(CO)(PPh3)2] + C6H6
[RhCl(CO)(PPh3)2] + C6H6
[RhCl(PPh3)3] + C6H13CHO ![]() [RhCl(CO)(PPh3)2] + [C6H12 + C6H14]
[RhCl(CO)(PPh3)2] + [C6H12 + C6H14]
Ethene is bound reversibly - but strongly enough for this coordinated ethene not to be reduced by H2 - forming [RhCl(C2H4)(PPh3)2]
[RhCl(PPh3)3] + C2H4 ![]() [RhCl(C2H4)(PPh3)2] + PPh3
[RhCl(C2H4)(PPh3)2] + PPh3
If H2 is coordinated first, though, ethene can be hydrogenated.
In this selection of reactions, RhCl(PPh3)3 can even be converted into a thiocarbonyl complex.
 Why Wilkinson?
Why Wilkinson?Geoffrey Wilkinson led the research group that pioneered this molecule and its applications. RhCl(PPh3)3 was actually prepared independently at much the same time in Martin Bennett's group at University College, London (Bennett had done the research for his PhD a few years earlier under Wilkinson's supervision). R.S. Coffey at ICI was also to discover the catalytic ability of RhCl(PPh3)3 in independent research.
Wilkinson shared the Nobel Prize for Chemistry with Ernst Otto Fischer in 1973, in the words of the citation "for their pioneering work, performed independently, on the chemistry of the organometallic, so called sandwich compounds". Wilkinson's work on ferrocene and other metallocenes was one of a number of highlights of his career, along with "ring-whizzer" fluxional molecules, transition-metal alkyls and metal imides, for example, along with the catalytic work on rhodium and ruthenium complexes.
Acknowledgements: Thanks are due to Martin Bennett and Fred Jardine, for helpful information, and to Richard Grainger for telling me about Ivermectin.
![]()
![]()