|

Wine Lactone
A key odorant, particularly in white wines.

Simon Cotton
University of Birmingham

Molecule of the Month September 2022
Also available: HTML version.

| 
Wine lactone is found in wine.
[Photo: Sanjay Acharya, CC BY-SA 3.0 via Wikimedia Commons] |
 |
|
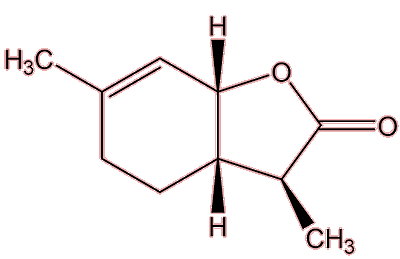
Wine lactone
(3S,3aS,7aR)-3,6-Dimethyl-3a,4,5,7a-tetrahydro-3H-1-benzofuran-2-one |
|
And where was it first discovered?
In the urine of koalas.
Surely, you're taking the...
No, koala urine actually. After they’d been fed a few eucalyptus leaves.
So why is it called wine lactone?
Well, twenty years after the koala experiments, the molecule was found to be present in some white wines, and to be a very important flavour ingredient, smelling like ‘coconut, woody and sweet’. It has also turned up in places like orange juice, black pepper, grapefruit juice and in some red wines. The molecule contains an ester group as part of the five-membered ring – that makes it a lactone (a cyclic ester).
|

[Photo: Diliff, CC BY-SA 3.0 via Wikimedia Commons |
Why hadn’t anyone discovered it before?
The molecule that turned up had a very low aroma threshold (the lowest concentration of a compound that can be smelt). That means that although it is only present in very small amounts in the wine (which made it hard for analysts to find), it has an important influence on the smell and taste of the wine. Helmut Guth, the German chemist who first found it in these wines, confirmed his identification of it by synthesising wine lactone.
How did he make it?
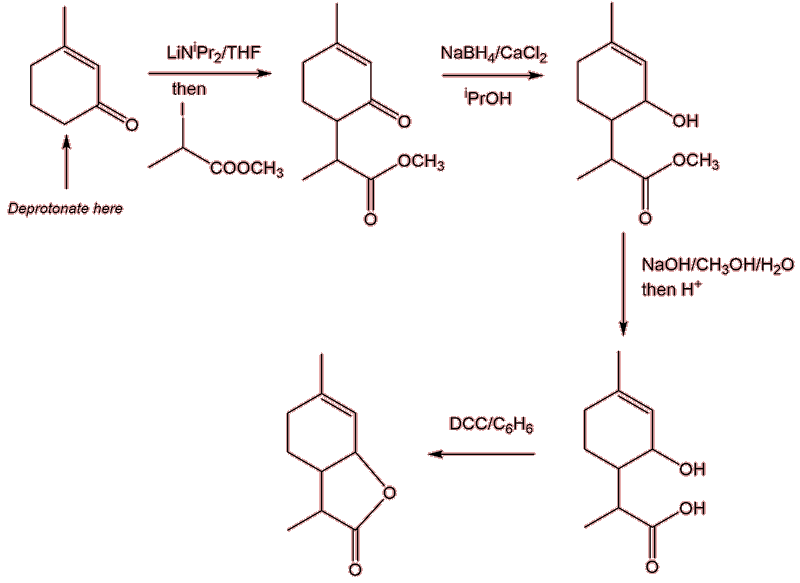
Guth chose methyl 2-iodopropanoate as one starting material, the other reagent being 3-methylcyclohex-2-enone. He used the very strong base lithium bis(isopropyl)amide, LiN(CHMe2)2 to deprotonate the ketone at its most acidic carbon, next to the polar C=O group. The carbanion formed carries out a nucleophilic attack on the methyl 2-iodopropanoate, displacing iodide, as a C-C bond is formed. This step generates the carbon skeleton required, the rest of the synthesis is concerned with forming the second ring. First, sodium borohydride is used to selectively reduce the carbonyl group to an alcohol. Next, the ester linkage undergoes alkaline hydrolysis, forming a carboxylate which, on acidification, yields the carboxylic acid. In the final step the carboxylic acid group can be esterified with the nearby alcohol group, generating the lactone linkage in the course of ring formation. The catalyst for the esterification reaction was N,N’-dicyclohexylcarbodiimide (DCC), a dehydrating agent more usually used in peptide synthesis.
So that’s it?
Matters are slightly more complicated than that. The ‘wine lactone’ molecule contains three asymmetric carbon atoms, which means that it has 23 stereoisomers; these 8 isomers exist as four pairs of enantiomers. Guth carried out his synthesis to confirm the structure he had assigned, and obtained a mixture of all eight. He was able to separate the mixture using gas chromatography with a column filled with a chiral stationary phase, and thus could get samples of each of the eight isomers, to see how they differed.
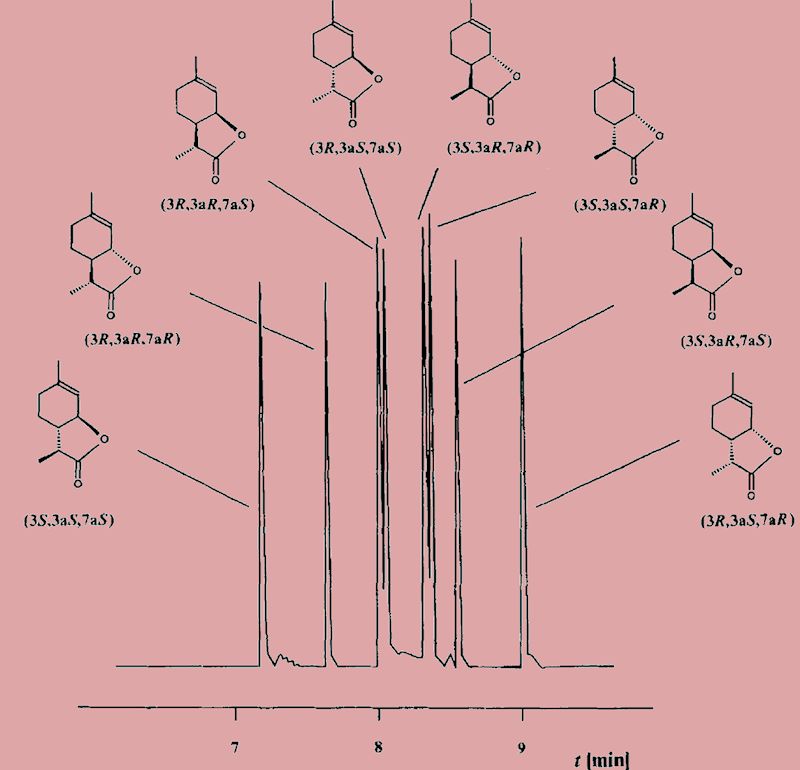
From: H. Guth, Helv. Chim. Acta., 1996, 79, 1559.
And what differences did he find?
Notably, that there was immense variation in their odour threshold, the minimum concentration of the substance that can be detected by smell. One isomer, the one that is generally found in white wine, has an exceptionally low odour threshold, around 10-5 mg per litre of air. Its enantiomer has an odour threshold 108 times greater.
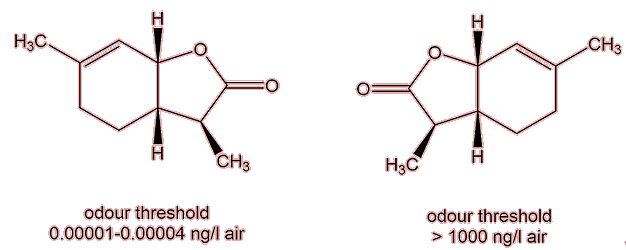
Is there just one isomer in wine?
Until recently, only the isomer with the lowest odour threshold, had been detected in wine, but in 2015 it was reported that its ‘less smelly’ enantiomer had been found in a four-year-old Chardonnay.
 |
 |
 |
Chardonnay grapes
[Photo: Saggittarius A,
CC BY-SA 4.0 via Wikimedia Commons] |
Shiraz grapes
[Verita at German Wikipedia,
CC BY-SA 3.0 via Wikimedia Commons] |
Cabernet Sauvignon grapes
[Photo: Agne27 at the English Wikipedia,
CC BY-SA 3.0 via Wikimedia Commons] |
How does it get into the wine?
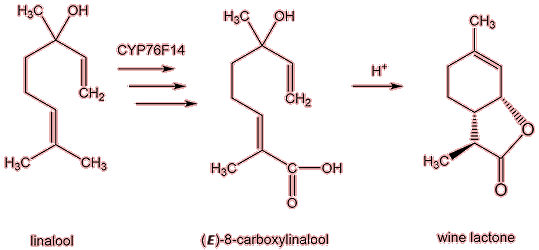
Wine lactone has not been found in grapes but is thought to be generated mainly as the wine matures. One possible formation route involves linalool (MOTM October 2013), which is present in the grape; linalool is known to be oxidised by a Cytochrome P450 enzyme CYP76F14 to (E)-8-carboxylinalool (a.k.a. menthiafolic acid), and this, in turn, is converted into to wine lactone in a very slow acid-catalysed reaction.
Is this typical of wine odorants?
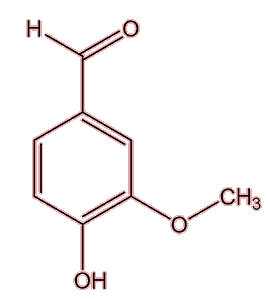
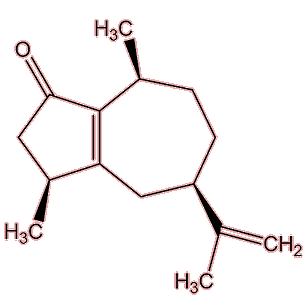
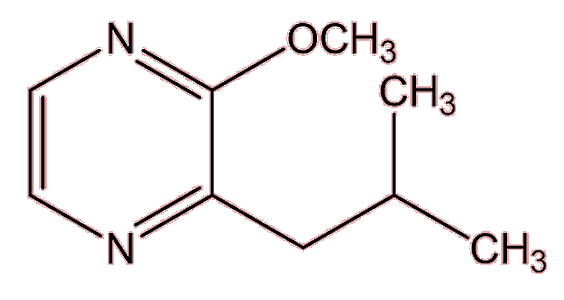
No, they have a variety of sources. Many esters are found in wines, generated after the ethanol has been formed in the fermentation process, but many of them have high aroma thresholds. 3-methylbutyl ethanoate (banana note) is one ester with a stronger smell. Several important molecules that give ‘character’ to a particular wine originate in the grapes used. Shiraz wines get their peppery aroma from a cyclic ketone named rotundone, which is also important to the smell of, well, peppercorns. Then 3-isobutyl-2-methoxypyrazine, is responsible for the green ‘bell pepper’ note of Cabernet Sauvignon and Sauvignon blanc wines also originates in the grape. Sometimes an important compound comes from the wood of the cask used to mature the wine, like the vanilla smell of vanillin (MOTM February 2008) or the woody, coconut note from β-methyl-γ-octalactone (‘oak lactone’).
 |
|
 |
|
| Vanillin |
|
Rotundone |
|
 |
|
| 3-isobutyl-2-methoxypyrazine |
|
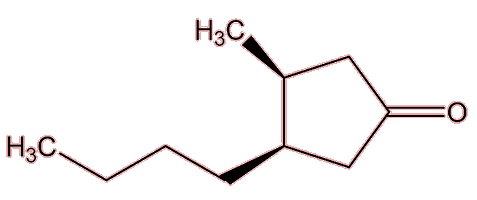 |
|
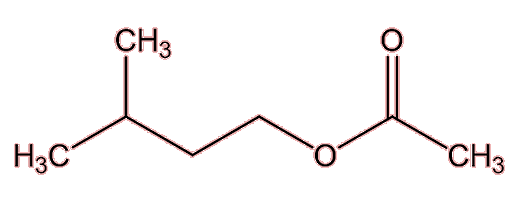 |
|
| Oak lactone |
|
3-methylbutyl ethanoate |
|
So there is a rich chemistry to wine flavour, unsuspected by the majority of drinkers, of course.

Bibliography
- CRC database Number: BJO63-G (general); BLO06-F (3R,4S,8S-form).
- I. A. Southwell, Tet. Lett., 1975, 24, 1885 (isolation of wine lactone from koala urine).
- H. Guth, Helv. Chim. Acta., 1996, 79, 1559 (stereochemical synth. and config. of wine lactone).
- H. Guth, J. Agric. Food Chem., 1997, 45, 3022 (wine lactone in Gewürztraminer and Scheurebe wines).
- H. Guth, J. Agric. Food Chem., 1997, 45, 3027 (odour activity values (OAVs) of wine lactone and other odorants).
- B. Bonnländer, B. Baderschneider, M. Messerer and P. Winterhalter, J. Agric. Food Chem. 1998, 46, 1474 (formation of wine lactone from monoterpenoid precursor in Riesling).
- A. Hinterholzer and P. Schieberle, Flavour Fragrance J., 1998, 13, 49-55 (wine lactone in Valencia Late Oranges).
- T. Jagella and W. Grosch, Z. Lebensm.-Unters. Forsch. A. Eur. Food Res. Technol., 1999, 209, 16. (wine lactone in black peppers).
- A. Buettner and P. Schieberle, J. Agric. Food Chem., 1999, 47, 5189 (wine lactone in grapefruit).
- R. Lopez, V. Ferreira, P. Hernandez and J. F. Cacho, J. Sci. Food Agric., 1999, 79, 1461 (wine lactone in red wines).
- S. P. Chavan, R. K. Kharul, A. K. Sharma and S. P. Chavan, Tetrahedron: Asymmetry, 2001, 12, 2985–2988 (simple synthesis of (−)-wine lactone).
- A. Högnadóttir and R. L. Rouseff, J. Chromatography A, 2003, 998, 201–211 (wine lactone in orange essence oil).
- J. Giaccio, D. L. Capone, A. E. Hakansson, H. E. Smyth, G. M. Elsey, M. A. Sefton and D. K. Taylor, J. Agric. Food Chem., 2011, 59, 660 (formation of wine lactone from grape-derived secondary metabolites).
- J. Giaccio, C. D. Curtin, M. A. Sefton and D. K. Taylor, J. Agric. Food Chem., 2015, 63, 8241 (formation of wine lactone from menthiafolic acid, and detection of second lactone isomer in aged wine).
- H. B. Wedler, R. P. Pemberton and D. J. Tantillo, Molecules, 2015, 20, 10781 (mechanistic aspects of precursor biosynthesis in wine grapes).
- T. Sato, R. Kobayakawa, K. Kobayakawa, M. Emura, S. Itohara, M. Kizumi, H. Hamana, A. Tsuboi and J. Hirono, Sci. Rep., 2015, 5, 14073 (human olfactory discrimination to wine lactone isomers).
- T. Ilc, et al., New Phytologist, 2017, 213, 264 (role of cytochrome P450 in formation of wine lactone).
- I.A. Ogunwande, A. A. Osunsami, S. E. Sotubo and O. A. Lawal, Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas, 2017, 16, 455 (wine lactone in antibacterial essential oil).


 Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.16726597]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.16726597]

![]()
![]()















