![]()
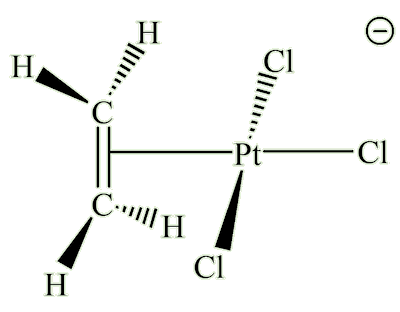
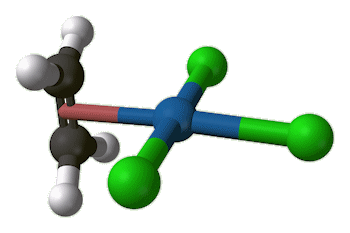
Zeise's salt
(K[PtCl3(C2H4)].H2O)
A pioneering organometallic
compound from 1830.
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month November 2021
![]()
  |
Zeise's salt(K[PtCl3(C2H4)].H2O)A pioneering organometallic
|
What’s the name for?Famous chemicals are often named after their discoverers. This is just such a case. William Christopher Zeise (1789- 1847) first studied the interaction between platinum chloride and ethanol in the mid-1820s, but it was in 1830 that he reported the compound that bears his name. He made the compound by boiling platinum (IV) chloride in ethanol, then adding potassium chloride, obtaining yellow crystals. (Zeise also made the corresponding ammonium salt). He established its constitution by chemical analysis, suggesting that the compound contained ethene. This was controversial, as the great Justus von Liebig suggested that an ethoxy (CH3CH2O-) group was present. But eventually Zeise was proven correct, when later workers confirmed his analyses. Zeise announced it in November 1830, and this appeared in print in 1831. Zeise’s original 1830 paper was delivered to the University of Copenhagen, and was published (in Latin) in the Anniversary Volume of the University of Copenhagen for that year. It was entitled :- “De chlorido platinae et alcohole vini sese invicem permutantibus nec non de novis substantiis inde oriundis.” |
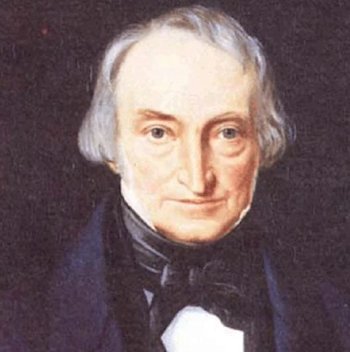 Painting of William Zeise (1789-1847). |
[Translator’s note: Alas!] The title is roughly translated as: ‘The reaction between platinum chloride and wine alcohol and on the new substances arising therefrom’. But he soon published his findings in German – at the time German chemistry was the most advanced in the world, and the important chemical journals were German ones. Zeise originally called the salt “sal kalico-platinicus inflammabilis” (inflammable potassium-platinum salt), whilst his German publication referred to it as “entzundliches Kali-Platin-Salz”. So “Zeise’s salt” is a bit easier to remember.
Some years after Zeise’s death, Birnbaum found the compound could be made directly from ethene, and obtained analyses that were a very good match for K[PtCl3(C2H4)].H2O. In 1934, J.S. Anderson repeated the synthesis and established that the ethene should be displaced by ligands like cyanide and pyridine, but did not clarify the structure otherwise. Subsequently a faster method was developed to make it from [PtCl4]2- and ethene, with SnCl2 catalyst; an even quicker method uses the reaction between K2PtCl4 and ethene in water: ethanol: conc. HCl mixture as solvent at 130°C, using microwave heating.
Although Zeise had established that ethene was present in the compound, it was not until the mid-20th century that both its structure was established and the bonding of the ethene was explained.
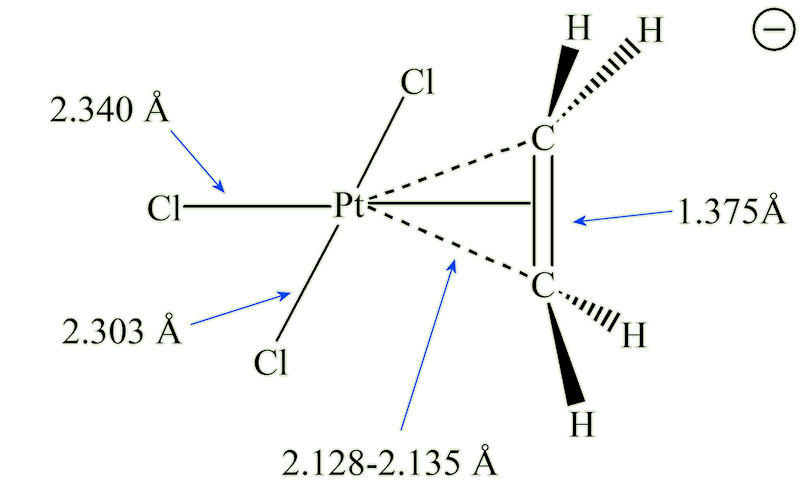
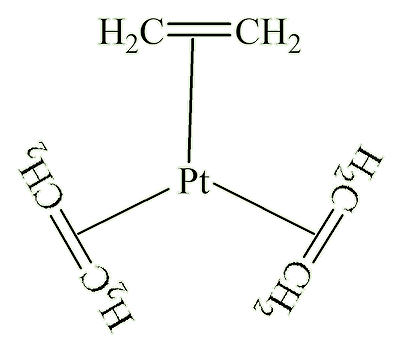
X-ray diffraction studies showed that the ethene molecule was bound side-on to platinum, and a slightly later neutron diffraction study located the hydrogen atoms, supplying some important details. The C=C distance, at 1.375 Å, is some 0.038 Å longer than in free ethene. The four hydrogen atoms are bent away from the platinum, with the carbon atoms 0.16 Å out of the plane of the four hydrogens.
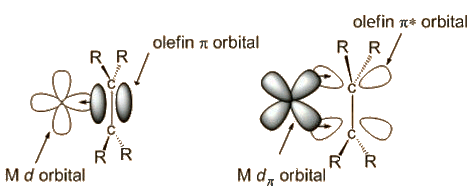
It was not until the 1950s that the bonding was explained by Chatt, Dewar and Duncanson, as follows:
It is the occupation of antibonding orbitals that weakens the carbon-carbon bond, and causes the lengthening of the C-C distance. The bonding at carbon changes, with some sp3 character, so the bond angle at carbon decrease below 120°, hence the hydrogens bending away from platinum.
Not at all: Birnbaum found that other alkenes like propene and pentene bind in a similar way. The chlorines can be substituted by other ligands like amines, as in trans-[Pt(C2H4)Cl2(NH3)]. One interesting species recently discovered is [PtCl3(N2)]-, detected in electrospray ionization mass spectrometry of K2[PtCl4] solutions. The Pt-N2 bond is weaker than the Pt-ethene bond in Zeise’s salt.
Far from it. Much of his research was on organic sulfur compounds. His greatest contribution was in the 1830s, when he discovered the (in)famously smelly thiols, analogues of alcohols with sulfur in place of oxygen, RSH. It was Zeise who coined their common name of mercaptans, because he found that they bound strongly to mercury (in Latin mercurium captans). Zeise pioneered Danish chemistry and was the first professor of chemistry in Denmark. He did all his research single-handed – there weren’t ‘research groups’ with postgraduate and postdoctoral students in those days – and he was his own ‘technical support’. He even did his own analyses.
It made chemists realise that metals could form bonds to organic groups, what we call organometallic compounds, a term coined by Sir Edward Frankland. He was a pioneer who made compounds like diethyl zinc and dimethyl mercury (MOTM October 2003) some 20 years after Zeise’s discovery. Discoveries flowed steadily. For example, in 1888, Ludwig Mond discovered nickel carbonyl, Ni(CO)4; the first methylplatinum compounds such as (CH3)3PtI were made by Sir William Pope in 1909; in 1951 ferrocene was reported. Its ‘sandwich’ structure (MOTM May 1996) spurred a huge expansion in organometallic chemistry, now an important part of the chemical industry.
Over a hundred years after Zeise, an important industrial process was developed to convert ethene into the aldehyde ethanal. This is known as the Wacker process, named after Wacker Chemie, the firm developing it. It uses palladium, in the form of palladium chloride, the lighter homologue of platinum, to oxidise ethene to ethanal. Over a million tons of ethanal are manufactured a year this way.
H2C=CH2 + PdCl2 + H2O 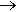 CH3CHO + Pd + 2HCl
CH3CHO + Pd + 2HCl
Apart from PdCl2, the process uses CuCl2, in combination with oxygen (from air) to reoxidise the palladium produced to PdCl2, thus regenerating the catalyst.
Pd + 2CuCl2 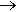 PdCl2 + 2CuCl
PdCl2 + 2CuCl
2 CuCl + ½ O2 + 2 HCl 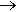 2CuCl2 + H2O
2CuCl2 + H2O
It is thought that ethene binds to palladium in a similar way to that in Zeise’s salt.
Indeed you can, with Pt(C2H4)3 as an example. This is a platinum(0) compound, and has a trigonal planar geometry, with the platinum and all six carbon atoms in the same plane.
And the future?Metal-based anticancer drugs, pioneered by cis-[Pt(NH3)(2Cl2)], cisplatin (MOTM August 2000) have been an important development in medicinal chemistry in the past half-century. These target DNA and although very successful in treating certain forms of cancer (e.g. testicular cancer) have their disadvantages. Recently some compounds have been made with a Zeise-type group linked by CH2 spacers (n = 1-4 in the diagram) to acetylsalicylic acid (a.k.a. aspirin, MOTM February 1996), shown in the diagram by the yellow background. |
 Aspirin tablets. [Photo: Ragesoss, CC BY-SA 4.0 via Wikimedia Commons] |
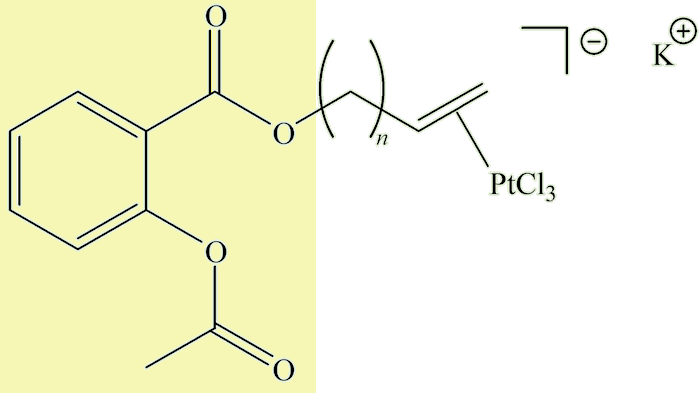
These have been designed to inhibit cyclooxygenase (COX) enzymes and are being studied as possible anticancer agents.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.15764199]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.15764199]