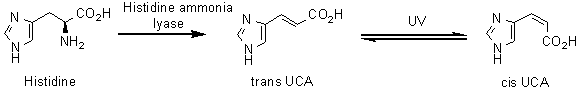|
Professor Varinder K Aggarwal Asymmetric Synthesis, Methodology, Natural Product Synthesis e-mail v.aggarwal@bris.ac.uk http://www.bris.ac.uk/Depts/Chemistry/staff/vaggarwal.htm |
Projects include the development of new catalytic processes for asymmetric synthesis; the use and application of reactive intermediates (carbenes, radical cations) for catalysis; the development of new methodology and its applications in synthesis, and total synthesis of biologically important targets.
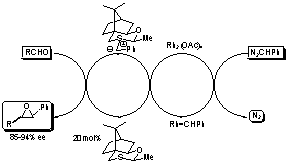 Catalytic Asymmetric Epoxidation of Carbonyl Compounds and Related
Reactions
Catalytic Asymmetric Epoxidation of Carbonyl Compounds and Related
Reactions
We have developed a catalytic asymmetric process for converting carbonyl compounds directly into epoxides and new chiral sulfides have been synthesised which give high asymmetric induction. Substitution of the aldehyde for an imine leads to aziridines and substitution with an electron poor alkene leads to cyclopropanes, again with high levels of asymmetric induction. Application of these new procedures to the synthesis of challenging synthetic problems together with the development of even more user and environmentally friendly methods for generating the metallocarbene will be explored.1
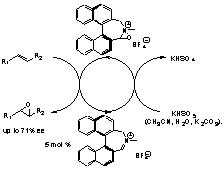 Amine/iminium Salt Catalysed Epoxidation of Alkenes
Amine/iminium Salt Catalysed Epoxidation of Alkenes
The development of chiral amines and iminium salts for catalytic asymmetric epoxidation of alkenes. Our aim is to improve the asymmetric induction and to develop new reactions based on the unusual observation that amines themselves can catalyse the same transformation.2
Total Synthesis of Biologically Important Targets
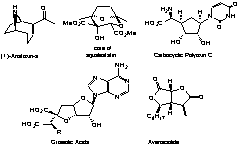 We
are interested in the asymmetric synthesis of biologically active compounds and
have developed short enantioselective routes to anatoxin A, the core of
squalestatin, the carbocyclic analogue of polyoxin C, a formal synthesis of
pyripyropene A; we are currently working on the total synthesis of griseolic
acid, avenaciolide and subglutinol. In each of these syntheses we either use
methodology that we have developed or new strategies that have not been used
previously with the particular class of molecule.3
We
are interested in the asymmetric synthesis of biologically active compounds and
have developed short enantioselective routes to anatoxin A, the core of
squalestatin, the carbocyclic analogue of polyoxin C, a formal synthesis of
pyripyropene A; we are currently working on the total synthesis of griseolic
acid, avenaciolide and subglutinol. In each of these syntheses we either use
methodology that we have developed or new strategies that have not been used
previously with the particular class of molecule.3
References
1. Synlett, 1998, 329; J. Am. Chem. Soc, 1996, 118, 7004; 1998, 120, 8328; Patents: WO95/11230, WO 98/51666; Angew. Chem. Int. Ed., 2001, 41, 8317
2. J. Chem. Soc., Chem. Commun., 1996, 191; Patent: WO 97/06147; J. Am. Chem. Soc., 2000, 1430; Patent: WO 99/01393
3. Angew. Chem., Int. Ed., 1999, 38, 1985; 2001, 41, 1433; J. Chem. Soc., Chem. Commun., 1994, 87; 1999, 325; J. Org. Chem., 1996, 61, 1192
Dr Kevin I Booker-Milburn New Synthetic Methods for Natural Product Synthesis e-mail k.booker-milburn@bris.ac.uk http://www.bris.ac.uk/Depts/Chemistry/staff/booker-milburn.html |
Research in our group is synthesis led and concentrates on the development of novel synthetic methodology for the construction of biologically active natural products.
The Development of Clean Methods for Radical Chemistry
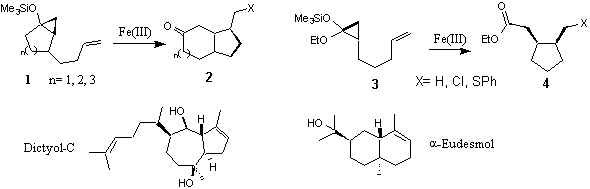
This involves the use of various Fe(III) species for the oxidative cyclisation of cyclopropanes and has resulted in the development of a new methodology for the synthesis of complex [n.3.0]bicyclo compounds (1→2) and highly functionalised cyclopentane esters (3→4) from readily available cyclopropylsilyl ethers. This methodology is currently being evaluated as a key step in the synthesis of natural products such as dictyol C and a-eudesmol.
New
Photochemical Methods for Natural Product Synthesis
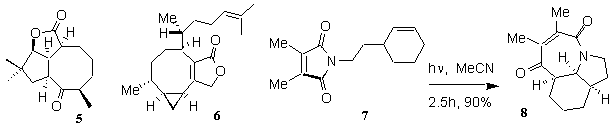
This has involved the development of new photochemical methods for the synthesis of cyclooctane-containing natural products. For example, a new photocycloaddition-fragmentation sequence was developed for the synthesis of asteriscanolide 5 and pachylactone 6. We are also investigating a fascinating photochemical [5+2] cycloaddition reaction for the synthesis of alkaloids (7→8).
References
J. Chem. Soc., Perkin Trans. 1, 1995, 2315
Tetrahedron Lett., 1998, 39, 7423
Eur. J.
Org. Chem., 2001,
1473
Org.
Lett., 2001, 3,
3005; 2002, 4, 1487
Dr Richard G Brereton Chemometrics applied to Modern Chromatographic and Spectroscopic Methods e-mail r.g.brereton@bris.ac.uk http://www.chm.bris.ac.uk/org/chemometrics/ |
Analytical chemistry is one of the main sources of industrial employment. A great deal of the advances over the past decade have been in the area of coupled chromatography and miniaturised spectroscopic probes. Together with these modern methods there is a need to process huge quantities of data by chemometric means. It is unlikely that a student will have had prior experience of all the techniques being used in the group.
We have access to superb instrumentation, such as three diode array HPLC machines dedicated to group use, various high field NMR machines, electrospray LCMS, GC, uv/vis. A typical project will involve learning the use of one or more such techniques and then developing chemometric methods for analysis of the data. Students have a variety of backgrounds, from hands-on analytical chemists to experienced programmers. Some students will be mainly interested in pure computing projects whereas others will want to gain hands-on experience of a variety of instrumentation together with experience of modern methods for chemometric data analysis.
A complementary interest is to develop cheap and fast methods of analysis such as spectroscopy and flow injection analysis. We are extending our applications to miniaturised on-line spectroscopic probes and will obtain an in-house mid IR probe. In such circumstances, the spectra of the relevant compounds are mixed up together and deconvolution or calibration is required to obtain the concentrations and kinetic information.
There are numerous applications, and some of current and recent interests are outlined below.
·
Pharmaceutical
analysis. Several projects
are underway with an element of industrial collaboration. In particular the use
of LCMS and LCNMR for impurity monitoring in process control is especially
important.
· Analysis of polyaromatic hydrocarbons. Originally sponsored by the Health and Safety Executive, we have employed a variety of different techniques such as UV spectroscopy, GCMS, LCMS and tandem MS to determine concentrations of these important pollutants.
· Rapid on-line reaction monitoring. Can reactions be followed in a quantitative way in real time? Use of flow injection analysis, uv/vis and MIR allows on-line kinetics. This is especially important for process control and optimisation in the pharmaceutical manufacturing industry.
· Pattern recognition of biological extracts. There is potential interest in using chemometrics to look at patterns in extracts e.g. from plants. This is important in areas such as metabolomics and also traditional herbal medicine such as from Asian countries. We have also worked on badger urine analysis. This contains chemicals that relate, for example, to sexual activity as well as diet, but there may be several hundred compounds.
· Forensic investigations. We are working on the analysis of complex chromatograms of urine samples in the area of forensic toxicology using combinations of coupled chromatography and chemometrics. We also have an interest in patent work using pyrolysis mass spectrometry.
· Characterisation of chromatographic columns. There is a huge number of columns on the market. Which is best for basic compounds, which for amino acids? Can a small number of tests be used to predict the performance of a new column?
References
Rapid Comm Mass Spec, 2001, 15, 135
Analyst, 2001, 126, 615
Anal. Chim. Acta, 2001, 447, 199
J. Chemom, 2002, 16, 125
Chemom Intell Lab Systems, 2002, 62, 61
Analyst, 2002, 127, 433 & 659; Talanta, 2002, 57, 721
See also The Alchemist (www.chemweb.com), Chemometrics "Features" articles
Mr Jim Carter Analytical Method Development and Forensic Chemistry e-mail jim.carter@bris.ac.uk http://www.bris.ac.uk/Depts/Chemistry/staff/jcarter.htm |
(1) The detection of trace quantities (picogram-microgram amounts) of controlled substances on banknotes is frequently used as part of the evidence to obtain a forfeiture order for suspected drug dealers. The presence of controlled substances in dust samples collected from clothing, cars, carpets etc. can also be used to elucidate criminal activities. Research has used techniques that can isolate a relatively pure extract of controlled substances from a sample (e.g. Solid Phase Extraction, SPE) or techniques that allow the direct analysis of complex mixtures such as dust or paper (e.g. Tandem Mass Spectrometry, MS/MS). The use of isotopically labelled compounds, such as d3 cocaine, has provided a ready means of quantifying the amounts present. Other work has examined mass spectrometry as a means to confirm results obtained from commercially available immunochemical test kits, increasingly used in roadside tests.
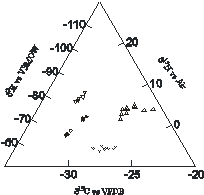 (2)
Current research is directed towards examining the stable isotopic composition
(2H, 13C, 15N) of controlled substances as a
means of linking, for example, traces found on clothing with bulk (gram)
quantities of seized material.
(2)
Current research is directed towards examining the stable isotopic composition
(2H, 13C, 15N) of controlled substances as a
means of linking, for example, traces found on clothing with bulk (gram)
quantities of seized material.

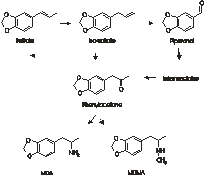
MDMA (3,4-methylenedioxymethylamphetamine) and MDA (3,4-methylenedioxyamphetamine) are both prepared from a number of naturally ocuring precursors and traded as “ecstasy”. Isotopic analysis of the active ingredient from five batches of ecstasy tablets revealed distinct isotopic characterists for each batch providing a chemical “fingerprint”. A combination of this technique and 2H-NMR also allows the synthetic route to be hypothesized.
|
(3) Pyrolysis is the breaking apart of large molecules into smaller ones using thermal energy. Pyrolysis-GC/MS is ideally suited to the analysis of macromolecular materials and has been applied to the characterisation of tablet formulations, both therapeutic and illicit. Data visualisation techniques are then used to generate “images” allowing the rapid comparison of tablets. |
|
References
Analyst 1999, 124, 103.
Analytical Chemistry 2000, 72, 11, 397A
Dr Russell J Cox Synthetic and Biological Chemistry: New Antibiotics e-mail r.j.cox@bris.ac.uk http://www.chm.bris.ac.uk/org/RJCox/Research.html |
Research in my group focuses on the development of new classes of antibiotics. Resistance to current antimicrobials has developed because most antibiotics are derived from natural sources. These compounds are produced in nature by organisms possessing resistance mechanisms which prevent the producing hosts from killing themselves. These resistance mechanisms can be spread by mobile genetic elements, eventually finding their way to pathogenic bacteria and causing previously treatable diseases, such as TB, to become untreatable. Our response to this problem is to develop and synthesise 'un-natural products'. We use two main strategies:
· Rational Design and Synthesis. We have targeted enzymes involved in bacterial cell wall biosynthesis. We use crystal structure data collected for these enzymes to help in the design of potential novel classes of antibiotics. Compounds such as 1 and 2 require new synthetic methodology to be developed. Compounds such as 3 and 4, synthesised by my group, are good enzyme inhibitors and we are now focussing on solid phase synthesis for the development of small libraries of new antimicrobial compounds.
· Directed Biological Synthesis. We use modern molecular biology techniques to access genes involved in antibiotic production, e.g. 5. These genes are then cloned and expressed and the resulting proteins, such as 6, are studied using the methods of mechanistic enzymology. With our understanding of their activities we have been able to use these recombinant proteins to synthesise organic compounds in vitro. Our aim is to be able to use proteins from diverse sources to synthesise new compounds to order.
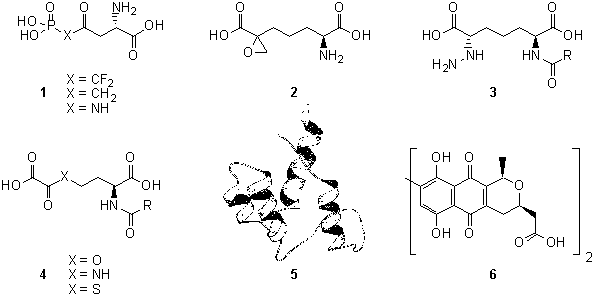
References
FEBS Letters, 1997, 405, 267; 1998, 433, 132
Chemistry and Biology, 1998, 5, 35; and, 699
Bio. Med. Chem. Lett., 1998, 8, 945; 2000, 8, 843
Nature, 1999, 401, 502
|
Dr John Crosby Enzymes, Structure, Function and Analysis e-mail john.crosby@bris.ac.uk http://www.bris.ac.uk/Depts/Chemistry/staff/jcrosby.htm |
Research in our group involves the use of a broad range of techniques including protein expression and purification, enzyme kinetics, and the accurate mass analysis of these large biomolecules by electrospray mass spectrometry. By isolating individual enzymes and establishing structural details, enzyme specificities, and kinetic characteristics we hope to understand fully how biosynthetic pathways are controlled.
Enzymology of Polyketide Biosynthesis
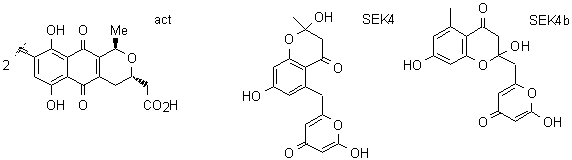
Many biologically active compounds are synthesised in nature via the polyketide pathway. The core of
all polyketide structures is similar, and is assembled by a multicomponent
enzyme system, the polyketide synthase (PKS). The synthase controls a series of
cycles of reactions in which simple building blocks (usually acetate) are
linked via carbon bond formation,
followed by a highly specific sequence of modifying reactions. We aim to
understand how the PKS controls the exact sequence of chemical steps involved
in the biosynthesis of antibiotics. This will be achieved by isolating
individual PKS components from a number of related bacterial systems, and
establishing structural details, enzyme specificities, and kinetic
characteristics. We will initially concentrate on the Streptomyces coelicolor system which produces actinorhodin (act)
and examine the production of related novel compounds such as SEK4 and SEK4b.
UV Induced Immunosuppression
The skin is a vital and dynamic constituent of the immune system, forming a protective boundary between the body and the external environment. It has been known for many years that exposure of animals to UVB results in suppression of some cell-mediated immune responses. The mechanism of this immunosuppression has not been fully elucidated, but is likely to involve a complex series of events initiated in the skin, and resulting in immune dysregulation. Urocanic acid (UCA), a major ultraviolet-absorbing component of the stratum corneum, is one of the immunosuppressive mediators currently of interest. One research aim is to examine the co-operative relationship between immunosuppressive mediators.
References
Nature, 1999, 401, 502 J. Photochemistry Photobiology, 1999, 48, 42
Chem. Biol., 1998, 5, 35, 699 Acta Dermato-venereologica, 1999, 79, 426
Professor Anthony P Davis Synthetic Supramolecular Chemistry e-mail Anthony.Davis@bristol.ac.uk http://www.bristol.ac.uk/Depts/Chemistry/staff/adavis.htm |
Our research is centred on supramolecular chemistry, “the designed chemistry of the non-covalent bond”. We aim to synthesize molecules which will act on others in controlled and predictable ways, hopefully with useful consequences. In much of our work we are trying to mimic nature. On the one hand we can help to explain the properties of natural molecules; on the other, their remarkable and complex behaviour suggests goals for the future. Some of our recent systems are shown below. 1 illustrates a design for anion receptors,1 based on a steroidal framework which features in much of our work.2 2 is another receptor, aimed at carboxylates, which shows useful enantioselectivity with certain a-amino acid derivatives.3 3 is a carbohydrate receptor which can extract glucose from water,4 while 4 is a “facial amphiphile” which promotes cell fusion and gene delivery.5 5 represents a “combinatorial library” of polymer-bound compounds from which we may be able to select “synthetic enzymes”.6 All these systems could have practical, especially medical, applications. For example, a glucose receptor like 3 could be used in a sensor which would greatly assist the management of diabetes.
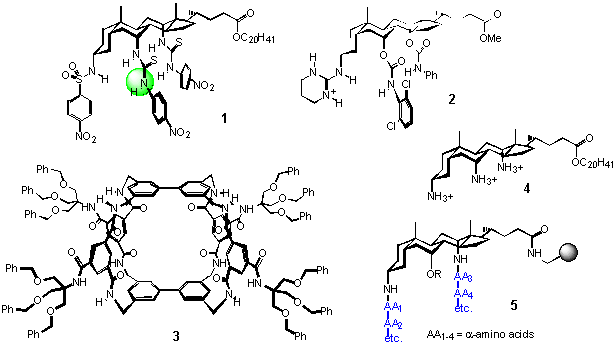
A project in our group would provide training in
organic synthesis, combinatorial chemistry, molecular modelling and the
measurement of supramolecular properties (e.g. binding constants). Much of our work is collaborative. At time of writing we belong to 3 EU-funded
Networks, affording opportunities for group members to build international
contacts and visit laboratories in other European countries.
References
1 J. Am. Chem. Soc., 2001, 123,
12716
2Comprehensive Supramolecular Chemistry,
ed. Y. Murakami, 1996, 4, 257
3 Chem. Commun., 1999, 9
4 Angew. Chem., Int. Ed., 1999, 38, 2979
Proc. Natl. Acad. Sci. USA, 2002, 99, 4863
5 J. Am. Chem. Soc., 2000, 122,
3252
6 Angew. Chem., Int. Ed., 2001, 40, 3813
|
Professor Richard P Evershed Biomolecular and Stable Isotopic Information in Modern and Ancient Environments e-mail r.p.evershed@bris.ac.uk http://www.chm.bris.ac.uk/org/evershed/ |
My research is inspired by a lifelong fascination with the natural world and the realisation that there are numerous opportunities to improve our understanding of the processes that shape both modern and ancient environments through chemical studies of biogenic organic molecules. All the research relies on the analytical capabilities of gas chromatography (GC) and high performance liquid chromatography (HPLC) in combination with mass spectrometry (MS), including isotope ratio MS. Only these techniques are able to provide the molecular scale resolution, structural and stable isotope information vital to studies of complex mixtures of organic compounds typically encountered in biological and environmental materials.
Current research focuses on three main fields:
· Archaeological chemistry. The primary aim of this research is to discover new ways of deriving chemical information from organic remains of archaeological interest with a view to improving our understanding of human activity in the past. The major study materials are pottery, human, animal and plant remains, anthropogenic soils and sediments. Major questions we answer relate to human diet, agriculture, exploitation of natural resources, and ritual and burial practices, e.g. mummification. The archaeological materials studied come from excavations in the UK, Europe, the Near East and the Americas, and cover a wide range of archaeological time periods, e.g. Neolithic, Minoan, Roman, Bronze Age, Iron Age, Saxon, Medieval, etc.
· Biogeochemistry and palaeoclimate reconstruction. This research focuses on studies of organic matter in soils, peat bogs and freshwater lake sediments. The research on soils involves studies of organic matter cycling, including its chemical characterisation, origin, transformation pathways and the role of soil dwelling organisms, including microorganisms and invertebrates. Research on peat and lake sediments is concerned with using distributions and stable isotope information contained in biomarker compounds as indicators of changes in past climate. Global warming is a central theme in this research area.
· Biomolecular palaeontology. This research is investigating the processes involved in fossilisation of invertebrates, higher plants, and mammals. The research is focusing on exceptionally well-preserved fossil and sub-fossil organisms, using a range of analytical chemical techniques to study the molecular fate of the major biochemical components of tissues, and the extent and longevity of their preservation over geological time. Studies of fossils are complemented by laboratory and field experiments aimed at exploring the mechanisms of preservation/degradation of biomolecules during fossilisation in real-time.
A project
carried out in this group will provide training and hands-on experience in
state-of-the-art chemical and instrumental techniques for studying the major
classes of biomolecules, i.e. lipids,
proteins, carbohydrates, DNA, lignin. Projects are cutting-edge and
complementary to other ongoing research in the group involving collaborators in
government research institutes, other universities and industry
References
Science, 1997, 276, 1541; 278, 432; 1998, 281, 402; 282, 147
Nature, 1985, 314, 528; 1997, 390, 667; 2000, 405, 175; 2001, 413, 837
Tetrahedron Letters, 1995, 36, 8875; 1997, 38, 8409; 1999, 40, 359
Professor Timothy C Gallagher Heterocyclic Synthesis and Methodology e-mail t.gallaher@bristol.ac.uk http://www.bristol.ac.uk/Depts/Chemistry/staff/tgallag.htm |
Research
in my group involves, as a central theme, the synthesis and reactivity of
important heterocyclic molecules. Many of our objectives and molecular targets
are of biological and medicinal significance, but we are also concerned with
understanding, as well applying and exploiting, reactivity and mechanism at the
more fundamental level. This inevitably
leads to new methodology both to solve specific problems and for more general
application.
Current Research Programmes:
· Chemistry/pharmacology of nicotinic agonists: novel drug structures of neurological significance, including UB-165;
· Directed deprotonation-transmetallation as a strategy for synthesis of heterocycles (pyridines) and the study of metal-mediated processes;
· The synthesis of C-linked carbohydrates (C-glycosides and C-glycopeptides) as probes for the structure and function of biologically interesting interactions; novel radical mediated synthesis of C‑glycosides based on 2-amino sugars as inhibitors of glycopeptide synthesis; novel scaffolds for carbohydrates as probes for the carbohydrate-carbohydrate interaction;
· Novel cycloaddition chemistry as a new strategy for the construction of lactam antibiotics;
· Total synthesis of natural products (such as cytisine and dysiherbaine) and tandem heterocyclisation reactions involving enantiomerically pure cyclic sulfates as double electrophiles;
Projects
are driven by the inherent challenge associated with the chemical problem and
also by the potential of the target molecule to address and solve more general
issues. We employ a wide range of
reagents and synthetic methods, and projects tackle a diverse set of
stereochemical problems. As a result,
we rely on spectroscopic and crystallographic analysis extensively. Several projects also involve collaborative
interactions with groups (both academic and industrial) outside Bristol, and
most postgraduate funding is based on CASE or fully funded studentship support
from the global pharmaceutical sector.
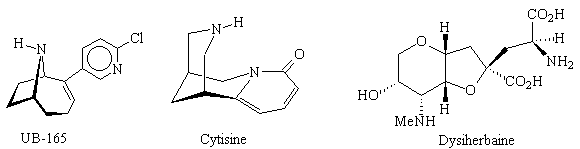
References
Bioorgan. Med. Chem. Lett. 1997, 7, 2867
Chem. Commun., 1998, 1723
J. Org. Chem., 1999, 64, 5453
J. Heterocycl. Chem., 1999, 36, 1365
Synlett, 2000, 1360; 2002, 808
J. Chem. Soc., Perkin Trans 1, 2000, 3047; 2001, 1270, 1281, 1897; 2002 in press
Organic Lett., 2000, 2, 4051; 2001, 3, 835
J. Neurosci., 2000, 20, 2783
J. Med. Chem., 2002, in press
Dr Guy C Lloyd-Jones Catalysis, Organometallics, Synthesis and Mechanism e-mail guy.lloyd-jones@bris.ac.uk http://www.chm.bris.ac.uk/org/LloydJones/ljgroup.htm |
The group has a diverse range of activities in areas that include physical-organic chemistry, organic synthesis, organometallic and coordination chemistry. In particular we are interested in asymmetric synthetic organic methodology, novel methods for catalyst discovery, organometallic structures, benyzne intermediates, ligand design and coordination chemistry.
The profound impact of organometallics on modern synthetic organic chemistry is indisputable. One of the key aims of the group is to use mechanistic insight (gained through a variety of modern physical-organic/inorganic techniques) to advance further the utility and application of organometallics - both as stoichiometric reagents and as catalysts. We are particularly interested in asymmetric catalytic processes.
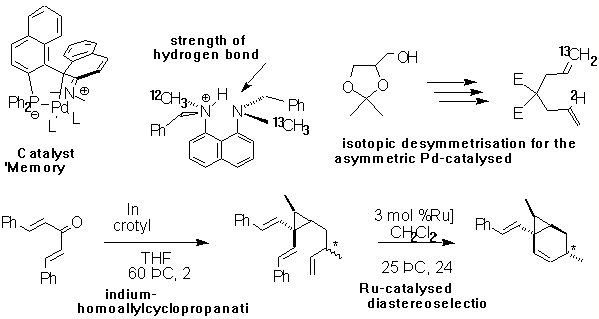
As a consequence of our diverse research activities, projects in the group encompass a wide range of synthetic and analytical techniques. General organic, organometallic and inorganic anaerobic/anhydrous techniques are used in the synthesis and purification of substrates for study. We make extensive use of the excellent high field NMR spectroscopic facilities available at Bristol [variable temperature, dynamic, multidimensional and multinuclear - 1H, 2H, 31P, 15N, 13C, 19F, 11B, etc.]. In conjunction with NMR spectroscopy we apply MS, IR, isotopic labelling (2H, 13C, 15N, 18O and 34S) and kinetic measurement techniques to the study of mechanism and structure. We have highly productive collaborations with other organic and inorganic research groups at other universities in Europe.
References
Angew. Chem. Int. Ed. Engl., 1998, 37, 1545 and 2001, 40, 2489 and 2002, 41, 953
J. Am. Chem. Soc., 1999, 121, 7714
Eur. J. Org. Chem., 2000, 975 and 2001, 1005
Chem. Eur. J., 1998, 4, 2539 and 2000, 6, 4451 and 2001, 7, 4205
Chem. Commun., 2000, 2447 and 2001, 187
Dr Martin Murray Analysis of Second-order NMR Spectra e-mail martin.murray@bris.ac.uk http://www.chm.bris.ac.uk/org/murray/mmurray.html |
The simple first-order splitting patterns in NMR spectra, as taught in the undergraduate course, are relatively easy to analyse, but never yield coupling constants between nuclei which are chemically equivalent (i.e. have the same chemical environment and therefore the same chemical shift). The first-order rules also fail to apply if either the chemical shifts are small compared to the coupling constants, or in symmetrical molecules where nuclei that are chemically equivalent are magnetically non-equivalent (i.e. have different couplings to a third nucleus in the molecule). In both cases, the resultant spectra are described as second-order, and are more difficult to predict or analyse. However, second-order spectra can often reveal coupling constants between chemically equivalent nuclei, which are unobtainable from first-order spectra.
While the effect of the first condition (small chemical shifts) can often be alleviated by using higher magnetic fields, the second condition (magnetic non-equivalence), which is very common even in simple organic molecules, e.g. o-dichlorobenzene, is unaffected by increasing the field strength. Second-order spectra are also common in both 1H and coupled 13C spectra of molecules that contain the 13C isotope, which makes up about 1% of natural carbon. Since ordinary 1H spectra are dominated by the 12C isotopomers, and 13C spectra are normally acquired with 1H decoupling, the second-order spectra are usually ignored, though with suitable analysis they can provide much useful chemical information.
Much of my research effort is directed at the measurement and analysis of such spectra. The former takes place on the School's high-resolution NMR spectrometers (see http://www.chm.bris.ac.uk/nmr/webs/facilities.htm), which are very powerful, and the analysis is usually performed using suitable programs on a PC. Suitable compounds are often available from other research groups in the School, or from a cooperative programme with the University of Düsseldorf in Germany, who make polyphosphonates with interesting NMR properties. Other projects might involve simple organic preparations, or alternatively study reactions taking place on a small scale in the NMR tube. The latter method has proved particularly successful in studies of phosphonitriles, compounds based on the P3N3 ring system.
References
Phosphorus, Sulfur, and Silicon, 1992, 65, 83, 1993, 77, 89
J. Chem. Soc., Dalton Trans., 1989, 21
Phos. Res. Bull., 1996, 6, 155
Dr Richard D Pancost Molecular Biogeochemistry e-mail r.d.pancost@bris.ac.uk http://www.bris.ac.uk/Depts/Chemistry/staff/rpancost.htm |
Molecular biogeochemistry is the study of the source, structure, and distribution of naturally occurring compounds (biomarkers) such that their presence in ancient or modern ecosystems can be used to elucidate processes or environmental conditions. Such research requires a variety of chemical extraction and degradation methods for sample preparation and the diverse analytical techniques necessary to identify such biomarkers (e.g. GC-FID, -FPD, -MS, -MS/MS; preparative GC; pyrolysis GC-MS; HPLC-MS). Moreover, advances in the past decade allow determination of the radiocarbon, stable carbon (d13C) and stable hydrogen (dD) isotopic compositions of individual biomarkers, which profoundly expands the environmental information contained in such compounds. This research is complementary to that of Professor Evershed and two areas of general interest are described below.
Proxies for Paleoclimate Reconstruction
Organic matter preserved in ancient marine, lacustrine, and peat deposits are vast repositories of information on Earth’s climate through time. Biomarkers provide insight into the organisms living in the past, and the environmental conditions necessary for such organisms to thrive can then be elucidated. Further information is provided by the isotopic compositions of such biomarkers. Because d13C and dD values of biomass are governed by environmental conditions, compound-specific isotope proxies can be used to reconstruct ancient pCO2 levels, rainfall, temperature, food web structure, and methane cycling. My research focuses on the development and refinement of such proxies by using cultures and field samples to identify diagnostic compounds and the controls on their isotopic compositions. I also am engaged in research that applies those techniques to specific problems in Earth history. Past, ongoing, or future research interests include investigations of climate change associated with mass extinctions, greenhouse periods, and the evolution of life. In particular, the capability for compound specific hydrogen isotope analyses (CSIHA) is very new and promises widespread utility; because the School of Chemistry is one of few institutions with the capacity for CSHIA, we expect to be at the forefront of this research.
Geomicrobiology
Bacteria and archaea comprise two of the three kingdoms of life and are essentially ubiquitous on the Earth’s surface. Recent developments in genetic techniques have reinvigorated the field by prompting new discoveries and reaffirming the importance of microbes in biogeochemical processes. At the same time, new analytical chemistry techniques now allow direct contributions to microbiology from molecular biogeochemists, particularly when novel biomarkers are integrated with isotopic determinations. My own work in this area includes studies of the microbiology of CO2 and methane cycling in peat deposits and the role of archaea in anaerobic methane oxidation. I also study the archaea and bacteria present in extreme environments, such as hydrothermal vents and deep-sea brine lakes. Profound interest in extreme settings arises from the potential that extremophiles could generate medicinal compounds and the prevailing impression that such settings represent good models for life on the early Earth.
References
Nature, 1999, 399, 342
Appl. Envir. Microbiol., 2000, 62, 1126
Org. Geochem., 1998, 29, 1649; 30, 319
Geology, 2001, 28, 663
Professor Thomas J Simpson Natural Product and Biological Chemistry e-mail tom.simpson@bris.ac.uk http://www.bris.ac.uk/Depts/Chemistry/staff/tsimpson.htm |
Natural products are of great interest as a result of the huge diversity of structures and the large range of biological activities they display. A large proportion of currently used drugs – antibiotics, anti-cancer compounds, immunosuppressive agents, etc. – are derived from natural products. Our group is involved in the study of all aspects of these: isolation and structure elucidation of novel compounds; synthesis of important target compounds, and, in particular, study of their biosynthesis – how they are assembled in the microorganisms (bacteria and fungi) that are the major source of these important compounds.
There are many facets to biosynthetic work – synthesis and incorporation of isotopically labelled intermediates, isolation and characterisation of the biosynthetic enzymes (which in turn lead to structural studies, e.g. protein NMR and X-ray, and mass spectrometry) and mechanistic studies.
The compounds we study – e.g. the important antibiotic pseudomonic acid, the cholesterol-lowering agent squalestatin S (1), the anti-fungal agent monocerin (2) – are all produced by the polyketide pathway. Our major aim is to understand how these compounds are produced so that we may tailor the biosynthetic pathway in a rational way to make new compounds with predictable changes in their structure and so produce new antibiotics and other pharmaceutical agents with new and improved properties.
The work, which involves a large multidisciplinary group, combines structural and synthetic organic chemistry, protein chemistry and biochemistry, microbiology and molecular genetics. Projects can involve one or more of these aspects and so provide a broad training in applications of organic chemistry to biological problems, experience of which is in high demand in the pharmaceutical and chemical industries. Our group is exceptionally well equipped for this work, and we have strong ongoing collaborations with other academic groups both in Bristol and elsewhere, and with a wide range of industrial laboratories.
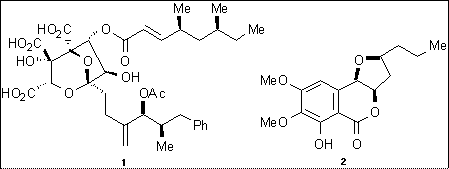
References
Structure: Tetrahedron Lett., 1999, 40, 133; Biochemistry, 1997, 36, 6000.
Synthesis: Tetrahedron Lett., 1997, 38, 5367, 1999, 40, 4093; Chem Commun., 2000, 1109
Biosynthesis: Chem. Commun., 1997, 2245, 1999, 1039.
Biochemistry: Chem. Biol., 1998, 5, 35, 699; Biochemistry, 2002, 41, 1421; Nature, 1999, 401, 502
Professor Chris Willis Natural Product Synthesis and Methodology e-mail chris.willis@bris.ac.uk http://www.bris.ac.uk/Depts/Chemistry/staff/cwillis.htm |
Natural products isolated from bacteria, fungi and plants are a rich source of compounds of medicinal and agrochemical importance. Their complex structures often require the development and use of mild and selective methods for their efficient synthesis and the application of a wide-range of spectroscopic techniques to elucidate the structures of intermediates on the synthetic pathway.
Our research centres upon the development of approaches to the enantioselective total synthesis of natural products. Current targets include, for example, barbamide and a series of unusual chlorinated diketopiperazines isolated from marine organisms as well as alkaloids, macrocycles such as clavosolide A and the antibiotic pseudomonic acid. Methods are being explored for the enantioselective synthesis of oxygen- and nitrogen-containing heterocycles for example using biotransformations and via ring forming reactions which enable the creation of up to three asymmetric centres in a single step. In addition, our research programmes encompass the design and synthesis of probes to elucidate biosynthetic pathways and enzyme mechanisms. These projects require the routine use of a range of spectroscopic and analytical techniques.
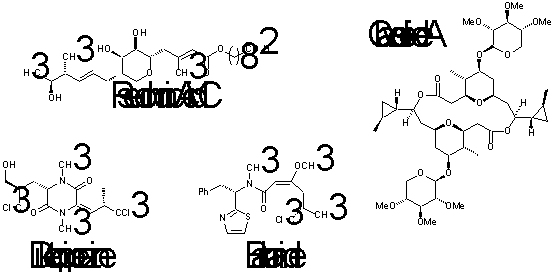
The majority of the research projects are in collaboration either with Industry or with scientists in other University Departments.
References
J. Am. Chem. Soc., 1998, 120, 7131
J. Org Chem., 1998, 63, 7764
Chem. Commun., 1999, 2049; 2000, 1109; 2001, 835, 1934
Org. Lett., 2002, 4, 577
J. Chem. Soc., Perkin Trans. 1, 2000, 43, 901, 2475, 3406
Tetrahedron
Letters, 1998, 39, 7415; 1999, 40, 1069, 4093; 2000, 41, 397, 8001
Tetrahedron, 2000, 56, 9103
Dr Paul J Wyatt Asymmetric Methods in Organic Chemistry e-mail paul.wyatt@Bris.ac.uk http://www.chm.bris.ac.uk/org/wyattgroup/layout/pjwHome.htm |
The syntheses in all of our projects involve a broad variety of modern organic chemical reactions and necessarily include many asymmetric techniques. The projects present all of the challenging facets of modern organic chemistry including stereochemistry, symmetry, NMR, chiral HPLC analysis and mechanisms.
C3 Symmetry
C2 Symmetry is already used extensively in the world of organic chemistry and has been strikingly successful at chiral induction because it can cut in half the number of different pathways through which a reaction proceeds. C3 Symmetry – another symmetry element of the tetrahedron – has the potential to cut by three, but is largely a fresh and unexplored topic. We are synthesising C3 symmetric chiral auxiliaries, reagents and generally exploiting its advantageous properties. We have recently made novel amine 1 by a new synthetic method. As well as application to asymmetric organic reactions, amine 1 is being studied in the field of crystal engineering. Our related phosphine 2 is being studied as an asymmetric ligand
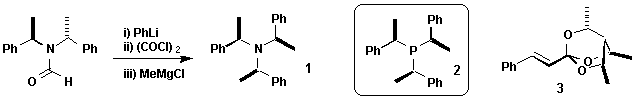
Other projects based upon C3 symmetry include the development of orthoesters 3 as chiral auxiliaries.
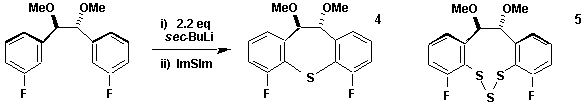
Seven Membered Heterocycles
Chiral seven membered rings are very useful in asymmetric synthesis. Sulfide 4 is readily synthesised in enantiomerically pure form. We are involved in the synthesis of similar rings with di- and trisulfide 5 bonds and with similar rings using boron as the heteroatom.
Alane
We have shown that alane is an excellent chemoselective reagent for reducing phosphine oxides to phosphines.
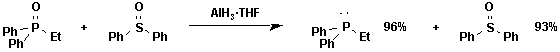
References
J. Chem. Soc., Perkin Trans. 1, 1999, 1095, 2000, 4222; 2001, 279
Tetrahedron Lett., 1998, 39, 4405; 1999, 40, 813, 5267