 Polyketide Biosynthesis
Polyketide Biosynthesis
Polyketide
Biosynthesis
Polyketides
are synthesised from simple precursors by iterative chemistry catalysed by
highly complex multi-functional enzyme systems, megasynthases known as
polyketide synthases (PKS). In brief, a PKS loads its substrates (acyl and
malonyl CoA) using an acyltransferase (AT) domain onto an acyl carrier protein
(ACP) and ketosynthase (KS) domains. The key carbon-carbon bond-forming step is
then catalysed by the KS, and subsequent programmed
modifications occur. In fungi, methyl groups are added by a C-methyl transferase (C-MeT) using S-adenosyl methionine (SAM). Selected keto-reduction (KR),
dehydration (DH) and enoyl reduction (ER) can then vary the functionality prior
to transfer back to KS for the next extension step. By iteration and variation
of these reactions, followed by subsequent post-PKS modifications of the
released product, fungi manufacture a vast array of secondary metabolites, many
of which display high chemical complexity and novel biological activity.
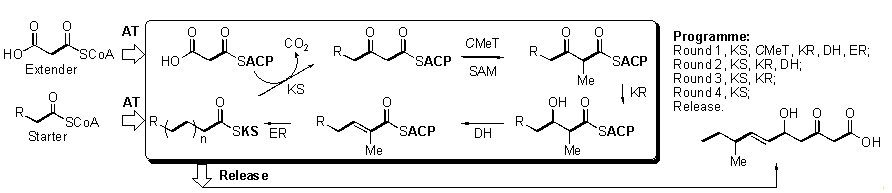
Scheme 1. Generic reactions catalysed by fungal PKS proteins. The hypothetical product would be produced by the programme shown.
The
Bristol
Polyketides Group work on three types of systems - (i) iterative Type I PKSs characteristic of
fungi, (ii) Type II PKSs responsible for the formation of a polycyclic aromatic
compounds in bacteria (actinomycetes) and (iii) a growing class (the “AT-less”)
of modular PKSs found in a number of diverse bacterial sources and responsible
for the biosynthesis of an increasing number of unusual antibiotics. Our early
investigations used isotopically labelled precursors (acetate, methionine etc) in feeding experiments.1 These were followed by sophisticated
experiments with complex synthetic putative precursors.2,3 We have developed new genetic methods for
the rapid cloning and expression of fungal PKS genes.4 We have also made pioneering advances in the
enzymology of the iterative Type II PKS involved in actinorhodin biosynthesis,5,6 e.g.
we have provided definitive evidence for the self-malonylation ability of Type
II PKS ACPs,7 and the decarboxylation of malonyl ACP by KS enzymes
in Type I and Type II synthases.8
Recently, we have sequenced the mupirocin gene cluster and investigated the
effect of targeted gene knockouts on the structure of mupirocin biosynthesis
intermediates.9 Fundamental to these key
advances has been the combination of
chemical synthesis, analysis, structure elucidation, molecular genetics,
enzymology and structural biology. We combine expertise in molecular
genetics, fungal biology and microbiology, polyketide biosynthesis in bacteria
and fungi (particularly of iterative systems), gene expression and protein
purification, chemical synthesis and analysis, natural products chemistry and
analytical chemistry.
1. Applications of Multinuclear
NMR to Structural and Biosynthetic-studies of Polyketide Microbial Metabolites.
T.J. Simpson, Chem. Soc. Rev., 1987, 16, 123-160.
2. Synthesis and incorporation
of the first polyketide synthase free intermediate in monocerin biosynthesis.
L.C. Axford, T.J. Simpson and C.L. Willis, Angew.
Chem. Int. Ed., 2004, 43,
727-730.
3. Synthesis of [1,2-13C2,15N]-L-Homoserine
and Its Incorporation by the PKS-NRPS System of Fusarium moniliforme into the Mycotoxin Fusarin C, D.
O. Rees, N. Bushby, R. J. Cox, J. R. Harding, T. J. Simpson and C. L. Willis, ChemBioChem, 2007, 8, 46-50.
4. Design and utility of
oligonucleotide gene probes for fungal polyketide synthases. T.P. Nicholson,
B.A.M. Rudd, M. Dawson, C. M. Lazarus, T.J. Simpson and R.J. Cox, Chem. Biol., 2001, 8, 157-178.
5.
MCAT is not required for in-vitro polyketide synthesis in a minimal
actinorhodin polyketide synthase from Streptomyces
coelicolor. A-L. Matharu, R.J. Cox, J. Crosby, K.J. Byrom and T.J. Simpson,
Chem. Biol. 1998, 5, 699-711.
6.
Dissecting the
Component Reactions Catalysed by the Actinorhodin Minimal Polyketide Synthase,
P. Beltran-Alvarez, R.J. Cox, J. Crosby and T. J. Simpson, Biochemistry, 2007, 46,
14672-14681.
7. Self-malonylation is an
intrinsic property of a chemically synthesized type II polyketide synthase acyl
carrier protein. C.J. Arthur, A. Szafranska, S. E. Evans, S.C. Findlow, S.G.
Burston, P. Owen, I. Clark-Lewis, T.J. Simpson, J. Crosby and M.P. Crump, Biochemistry, 2005, 44, 15414-15421.
8. A chain initiation factor
common to both modular and aromatic polyketide synthases. C. Bisang, P.F. Long,
J. Cortes, J. Westcott, J. Crosby, A-L Matharu, R.J. Cox, T.J. Simpson, J.
Staunton and P.F. Leadlay, Nature,
1999, 401, 502-505.
9. Accumulation of Mupirocin H
and Mupiric Acid During In vivo
Mutational Analysis of the Mupirocin Gene Cluster Reveals Labile Points in the
Biosynthetic Pathway: the ”Leaky Hosepipe” Mechanism, J-e Wu, Y. O’Connell, J.
A. Shields, R. J. Cox, J. Crosby, J. Hothersall, T. J. Simpson, C. M. Thomas
and C. L. Willis ChemBioChem, 2008, 9, 1500-1508, and references therein.