Dr. Richard Lonsdale
The cytochrome P450 enzymes (CYPs) are a family of haem-containing enzymes that play an important role in drug metabolism. CYP-mediated drug metabolism is carried out in the human liver by several different members of the CYP family (isoforms), most commonly involving insertion of a single oxygen atom (from molecular oxygen) into the substrate.
Generally, the metabolism of drugs by CYPs has a beneficial effect, resulting in the formation of soluble metabolites, which can be removed from the body, preventing accumulation of a particular substance to a toxic level. Complications (in some high-profile cases) can result in loss of life and arise due to the following factors:
-
1.Formation of toxic metabolites, such as epoxides, which can cause adverse drug reactions.
-
2.Different CYP isoforms have preferences for different drugs, and some isoforms react with the same drug, but with different chemo- and regioselectivities.
-
3.Metabolism of one drug can interfere with the metabolism of another, if they react with the same isoform.
QM/MM methods are helping in better understanding the factors that control the reactivity and specificity of CYP-mediated drug metabolism. These methods allow the active haem species (which has not been observed experimentally due to high reactivity) and substrate to be modelled with a QM method (such as B3LYP density functional theory), and the enzyme environment to be taken into account using molecular mechanics.1 This information should help in the development of new reactivity-based models, to predict the metabolites that are formed during CYP-mediated metabolism of new drug candidates.
The chemoselectivity of alkene oxidation is an important problem to address, as many drug molecules contain double bonds, and it can result in the formation of epoxides. The epoxidation and hydroxylation of cyclohexene and propene by the bacterial P450cam isoform have been modelled with QM(B3LYP)/MM methods. Structures for QM/MM modelling were chosen from molecular dynamics trajectories, to sample the different conformations of the enzyme-substrate complex. The energy barriers obtained for these processes are in qualitative agreement with experimental work, supporting the use of QM/MM methods in the study of selectivity for this class of enzyme. This work highlights the complexity involved in modelling these systems with QM/MM and the importance in the selection of starting geometries.
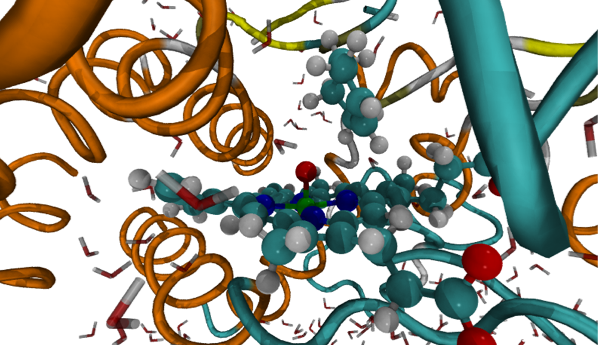
Reactivity and selectivity of cytochrome P450 enzymes from QM/MM modelling
This work is a collaboration with Professor Jeremy Harvey - http://www.chm.bris.ac.uk/pt/harvey/jeremy.htm
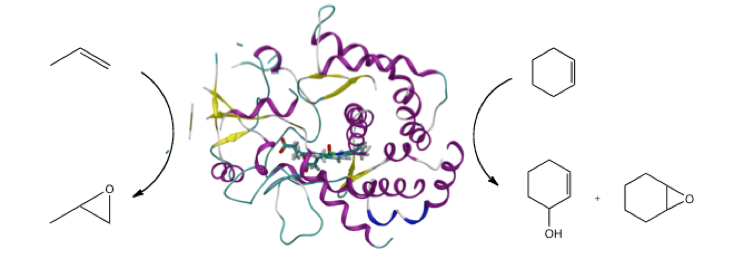
Chemoselectivity of alkene oxidation by P450cam2
Publications:
1. Lonsdale, R.; Harvey, J.N.; Mulholland, A.J., Effects of Dispersion in Density Functional Based Quantum Mechanical/Molecular Mechanical Calculations on Cytochrome P450 Catalyzed Reactions. J. Chem. Theory Comput. 2012, 8,4637.
2. Lonsdale, R.; Hoyle, S.; Grey, D.T.; Ridder, L.; Mulholland, A.J., Determinants of Reactivity and Selectivity in Soluble Epoxide Hydrolase from Quantum Mechanics/Molecular Mechanics Modeling. Biochemistry, 2012, 51, 1774.
3. Lonsdale, R.; Harvey, J.N.; Mulholland, A.J., A practical guide to modelling enzyme-catalysed reactions. Chem. Soc. Rev., 2012, 41, 3025.
4. Lonsdale, R.; Harvey, J.N.; Mulholland, A.J., QM/MM Studies of Cytochrome P450 Systems: Application to Drug Metabolism, in Iron-Containing Enzymes: Versatile Catalysts of Hydroxylation Reactions in Nature, Royal Society of Chemistry, 2012, pp. 366-399.
5. Lonsdale, R.; Olah, J.; Mulholland, A.J.; Harvey, J.N., Does Compound I Vary Significantly between Isoforms of Cytochrome P450? J. Am. Chem. Soc, 2011,133, 15464-15474.
6. Lonsdale, R.; Harvey, J.N.; Manby, F.R.; Mulholland, A.J., Comment on "A stationary-wave model of enzyme catalysis" by Carlo Canepa, J. Comput. Chem. 2011, 32, 368-369.
7. Lonsdale, R.; Ranaghan, K.E.; Mulholland, A.J., Computational Enzymology, Chem. Commun., 2010, 46, 2354.
8. Lonsdale, R.; Harvey, J.N.; Mulholland, A.J., Compound I reactivity defines oxidation selectivity in cytochrome P450cam, J. Phys. Chem. B, 2010, 114, 1156.
9. Lonsdale, R.; Harvey, J.N.; Mulholland, A.J., Inclusion of Dispersion Effects Significantly Improves Accuracy of Calculated Reaction Barriers for Cytochrome P450 Catalyzed Reactions, J. Phys. Chem. Lett., 2010, 1, 3232.
10. Green, J.C.; Herbert, B.J.; Lonsdale, R., Oxidative addition of aryl chlorides to palladium N-heterocyclic carbene complexes and their role in catalytic arylamination. J. Organomet. Chem., 2005, 690, 6054.
I was awarded an MChem in Chemistry from the University of Oxford in 2005. I first became interested in computational chemistry during my undergraduate project with Professor Jennifer Green, when I modelled organometallic reaction mechanisms. I subsequently came to Bristol in 2005 to study for my PhD with Professor Mulholland, which I completed in 2009. It was during this time that became interested in studying enzyme mechanisms. I am now a post-doctoral researcher in Professor Mulholland's group. My main area of research is in understanding the causes of reactivity of cytochrome P450 enzymes, however I also work on other enzymes such as epoxide hydrolase and heme dioxygenases.
