Molecular
Modelling
Molecular
Mechanics – empirical potential functions (continued)
Van der Waals Interactions
·
These consist of an attractive (dispersion)
component, and a short-range repulsive component often called ‘exchange
repulsion’.
·
The Lennard-Jones 12-6 potential is often used
in molecular mechanics potential functions to model van der Waals interaction
energy between two atoms separated by a distance r:
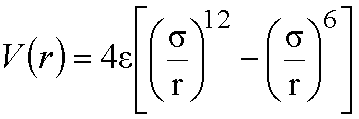
·
This function contains two parameters: the well
depth, e (the
depth of the energy minimum for the interaction), and the collision diameter, s (the separation at
which the interaction energy is zero).
·
Where the interaction is between atoms of
different types, e
and s must be calculated by
'mixing' the parameters of the two different atoms.
Dispersion
interactions
Dispersion
interactions are attractive, with the attractive force being due to
instantaneous dipoles arising from fluctuations in the molecular electronic
distributions.
A simple model, known
as the Drude model, for oscillating dipoles, predicts an interaction energy
proportional to:
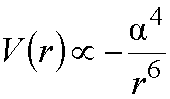
where a is the polarizability
of the molecules, and r is their
separation, i.e. the attractive energy is proportional to 1/r6,
which is reflected in the attractive component of the Lennard-Jones 12-6
potential.
Repulsive
interactions
·
At very short distances, molecules repel each
other strongly.
·
The forces increase very rapidly at small
internuclear distances, but are insignificant at larger distances.
·
Contributions to the repulsion include
nuclear-nuclear repulsion and electron-electron repulsion, as well as changes
in electronic kinetic energy.
·
The repulsive interaction is often described as
being due to (Pauli) exchange forces – because of the Pauli Principle,
electrons of the same spin cannot occupy the same region of space. This reduces
electron density between the nuclei, leading to an increase in nuclear-nuclear
repulsion.
·
In the Lennard-Jones 12-6 potential, the
repulsion is modelled by a 1/r12 term. A better description of the
repulsion is given by an exponential term, i.e. a repulsive interaction of the
form:
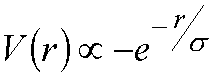
e.g. the
Buckingham potential has an exponential repulsive form (Leach pg. 209)