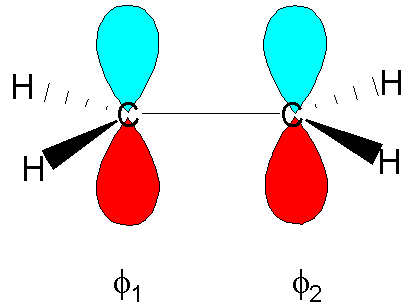
Finding
the p molecular orbitals of ethane using the LCAO approximation
The expression for the energy can be written as:
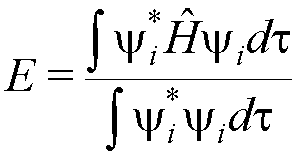
(this is the energy according to quantum mechanics: this energy is the ‘expectation value’ of
the energy operator (Hamiltonian operator) for any wavefunction; in this case yi is a molecular orbital of ethene).
The integrals
are over all space. The energy
calculated in this manner will depend on the coefficients ci1, and ci2
The variational
principle states that if an approximate wavefunction (such as this) is used to
calculate the energy, the value obtained for the energy is always greater than
or equal to the exact ground state energy of the system, i.e.
E ³ Eexact
The Variation Principle (Atkins 7th
ed. pg. 429)
(This was covered in Prof.
Balint-Kurti’s lectures on Quantum Concepts)
If
an arbitrary or approximate wavefunction is used to calculate the energy of a
system then the value obtained is always greater than or equal to the exact
ground state energy of the system.
This principle can be stated
mathematically as:
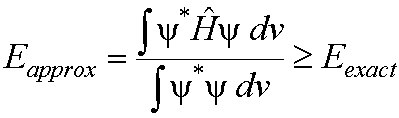 (1)
(1)
Another way of saying this is
The
energy of an approximate wavefunction is never less than the true energy
The Variation principle is the key to
finding wavefunctions of molecules.
It leads to
the Variational Method:
The Variational Method:
This is a method, based on the variational
principle, for finding approximate wavefunctions.
It is central for finding electronic
wavefunctions for molecules and enables us to calculate molecular orbitals,
molecular electronic wavefunctions and potential energy curves and
surfaces.
The method works as follows:
1. Construct an approximate wavefunction y which depends
on some parameters ci.
2. Calculate the energy using equation (1). The energy calculated in this way, E(ci),
will depend on the parameters ci
which are part of the approximate wavefunction.
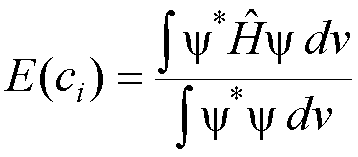 (2)
(2)
3. Choose
the parameters ci so as to minimize the energy
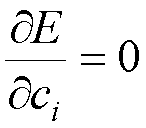 (3)
(3)
·
This minimized energy is still above the true ground state
energy.
·
The wavefunction with this set of parameters is the best
wavefunction of the form which has been chosen.
·
It
gives the lowest possible energy, as close as possible to that of the exact
ground state wavefunction.
So, for the p MOs of ethene:
·
The next stage in this case is to minimize the energy with respect
to the cip,, i.e, ci1 and ci2.
·
This minimized energy will still be above the true ground state
energy.
·
The wavefunction with the values of ci1 and ci2
that give the minimum energy is the best wavefunction it is possible to
obtain using just a simple combination of two atomic orbitals.
·
It gives the energy closest to the exact ground state energy.
As
you have seen in Prof.
Balint-Kurti’s lectures this leads to secular equations:
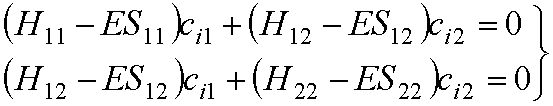
We can write them in matrix
form:
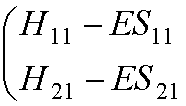
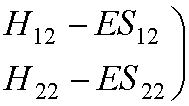
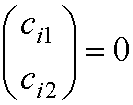
This leads to the secular
determinant
The ‘trivial’ solution of these
secular equations is
ci1
= ci2 = 0.
(Why is this of no interest?)
Next: the secular determinant and the
energies of the molecular orbitals of ethene