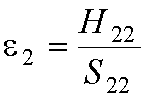A ‘nontrivial’
solution exists only if
det(H - ES)
= 0,
i.e. if the
determinant
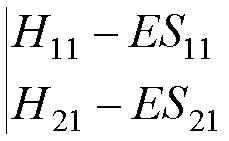
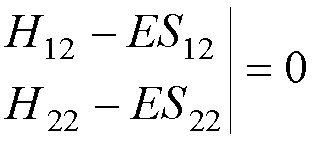
This
determinant is known as the SECULAR
DETERMINANT.
The
equations use shorthand for the Hamiltonian integrals (‘matrix elements’), i.e.
energies:
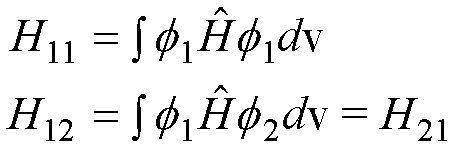
and
the overlap integrals/matrix elements (the overlap is a number between 0 and
1):
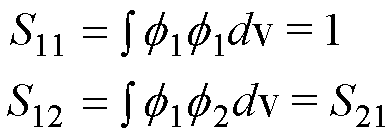
Note the symmetry H12= H21and S12= S21 in
these matrix elements.
Expanding the
secular determinant, we get a quadratic equation for E:
(H11 - ES11)(H22
- ES22) - (H12 - ES12)2 = 0
· This means
that there will be two values of E
which satisfy this equation.
· These two
values are the energies of the two
molecular orbitals and the index “i” has been used to label them.
· We denote the
two molecular orbital energies as E1
and E2.
· In our
example, one will be the bonding, the other the antibonding p-orbital
formed by the combination of the two C 2p orbitals.
The original atomic orbital energies, e1 and
e2, are:
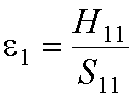 and
and