Estimating the
value of b from correlations with experimental data
To find the value of b, the resonance
(or bonding) integral (Hrs), we can compare the
predictions of Hückel Theory with experimental data.
Benzene and resonance energies
Benzene exhibits many unusual properties, for example it is more
stable than expected, e.g. comparing enthalpies of hydrogenation with that
expected for three double bonds.
For cyclohexene:
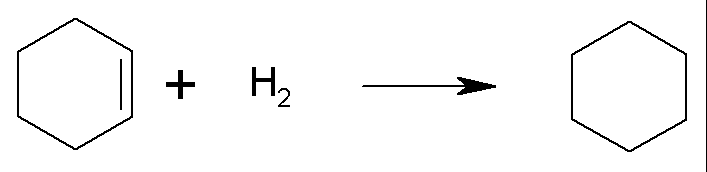
the enthalpy of hydrogenation is DH = –119.7 kJ/mol
For 1,3-cyclohexadiene:
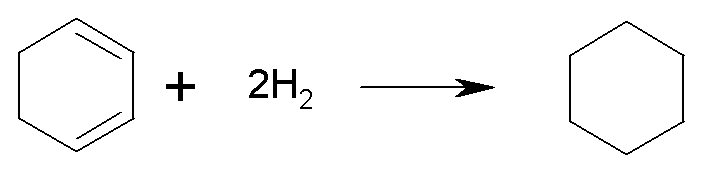
the enthalpy
of hydrogenation is DH = –231.8
kJ/mol. This is slightly less than twice the value for cyclohexene, so
1,3-cyclohexadiene is slightly more stable than expected (by 7.6 kJ/mol).
For benzene:
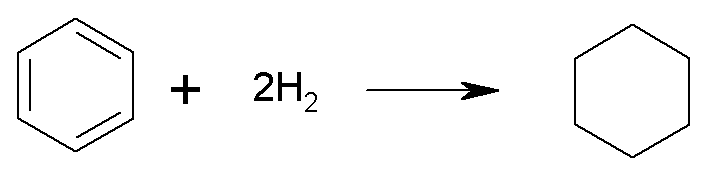
the enthalpy of hydrogenation (DH = –208.4
kJ/mol) is much less negative than expected for 3 double bonds (3´–119.7kJ/mol =
–359.1kJ/mol).
It is more stable than expected by (359.1 –208.4) = 150.7 kJ/mol
(36 kcal/mol).
This extra stabilization energy is described as the (experimental) ‘resonance
energy’ of benzene.
·
Hückel Theory gives the delocalization energy of benzene as 2b, therefore we can estimate the value of b as –75.35 kJ/mol.
(Notice this is much lower than the value of b mentioned earlier – the reasons for this are discussed later).