Alternative models for
understanding pericyclic reactions: aromaticity of transition state structures
[4+2], [8+2] and
[6+4] thermal cycloadditions are common
[2+2], [4+4] and
[6+6] cycloadditions are known almost only as photochemically induced reactions
(these numbers
refer to the number of electrons involved, e.g. the Diels-Alder reaction is a
[4+2] cycloaddition, with 4 p-electrons from the diene and 2 from
the dienophile)
The allowed
reactions involve (4n+2) electrons – the same ‘magic’ numbers as for
aromatic rings.
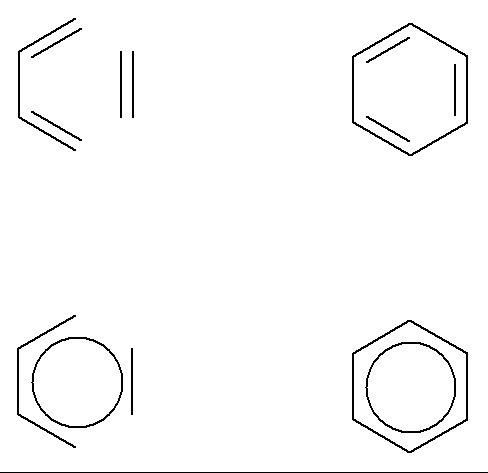
So we can think of the transition state structures for these reactions as being aromatic and so being stabilized,
e.g. the Diels-Alder transition state in some ways resembles benzene.
Transition state
structures with 4n electrons are avoided – except when they have an
antarafacial component.
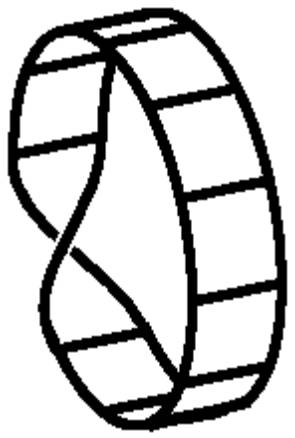
The p-orbitals in
a cyclic conjugated system like this will be form a Möbius strip if the
molecule has a single twist – possible in long, flexible molecules.
Calculations
predict that Möbius conjugated systems will be stable (aromatic) with 4n
electrons. Möbius strip (twisted) transition states with 4n electrons
are formed in some pericyclic reactions.
Recently, the first stable Möbius strip molecule was synthesized:
‘Synthesis
of a Möbius aromatic hydrocarbon’ D. Ajami et al. Nature 426,
819-821 (18 December 2003).