Alternative models for understanding pericyclic reactions: frontier
orbitals
A straightforward and useful way of deciding whether pericylic
reactions are allowed is to look at the interactions of the frontier orbitals
of the reactants.
These are the highest occupied molecular orbital (HOMO) of one
component and the lowest unoccupied molecular orbital (LUMO) of the other
reacting component.
For example, for the dimerization of ethene there is unfavourable
(antibonding) overlap between the HOMO of one molecule with the LUMO of the other.
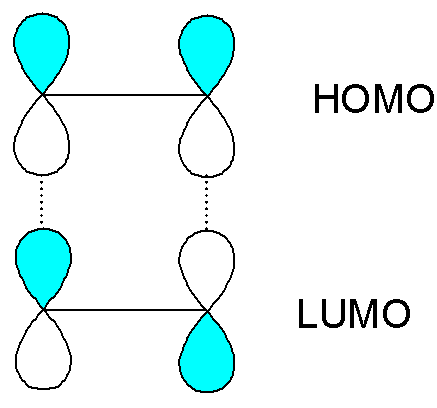
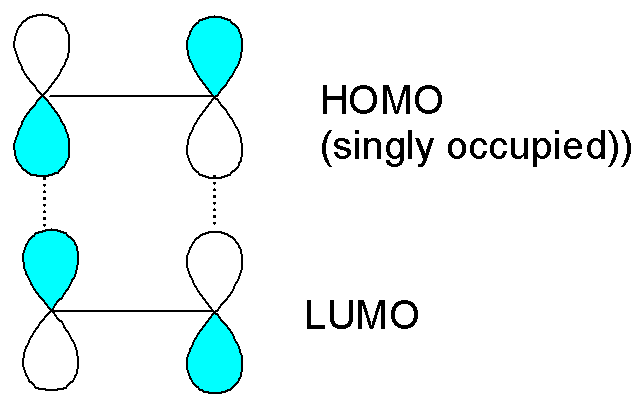
For the photochemical
cycloaddition of ethene, one molecule has had an electron excited from the HOMO to
the LUMO. The interaction between the HOMO (singly occupied) of the excited
molecule and the LUMO of the other ethene is now favourable:
For the Diels-Alder
reaction of butadiene with ethene, the interaction of the HOMO of each
component with the LUMO of the other is favourable:
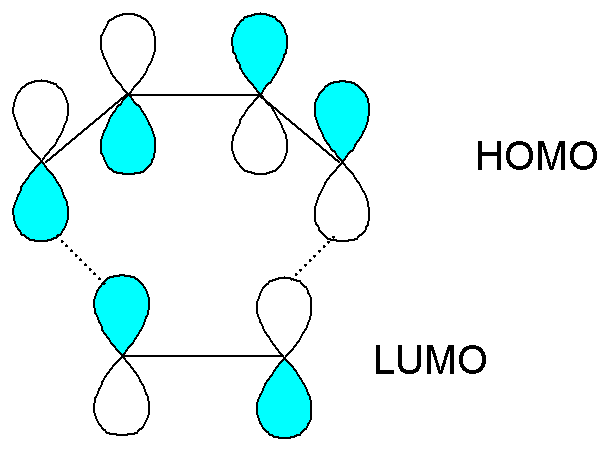
The interaction
is favourable with either set of frontier orbitals (both interactions should
therefore help the reaction to occur):
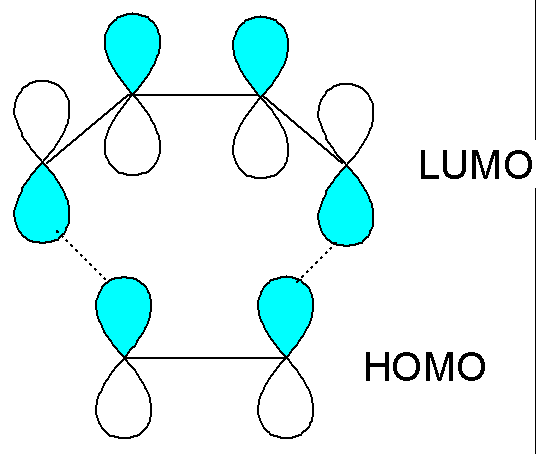
The frontier orbital approach works well at explaining many cycloadditions, but is more difficult to use for unimolecular reactions such as electrocyclic reactions (e.g. cyclobutene ring opening).
Examining frontier orbitals is more useful for understanding and
predicting small differences in reactivity, e.g. stereoselectivity, substituent
effects regioselectivity in Diels-Alder reactions.