Looking at the
shapes of the frontier orbitals allows many organic reactions to be understood.
The HOMO of one reactant will try to achieve maximum overlap with the LUMO of
another.
The LUMO of acetone shows the carbonyl carbon to be the likely site
of nucleophilic attack
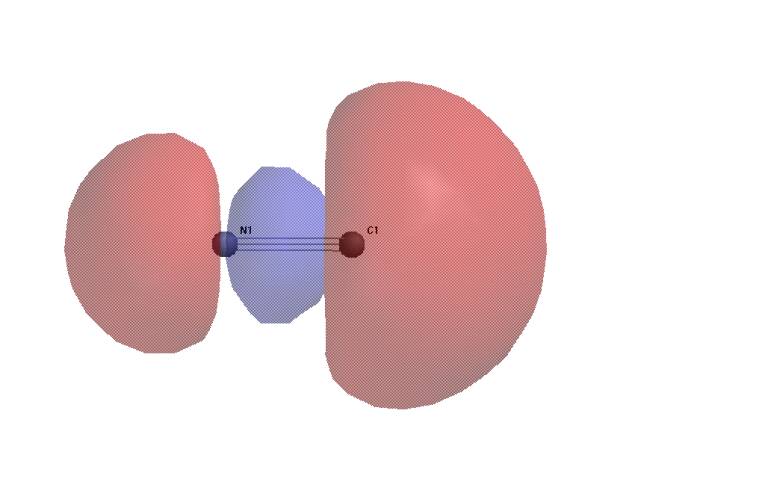
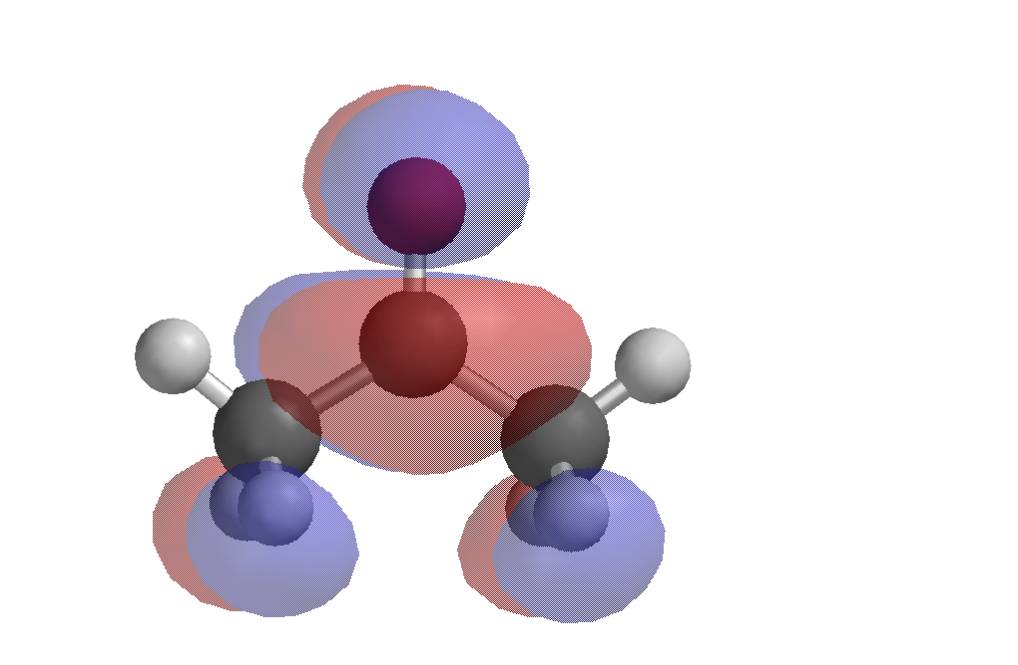
The HOMO of cyanide ion shows the carbon atom to be more likely to act as a nucleophile than the nitrogen (e.g. methyl cyanide is the major product of the reaction with methyl iodide; small amounts of methyl isocyanide are produced).
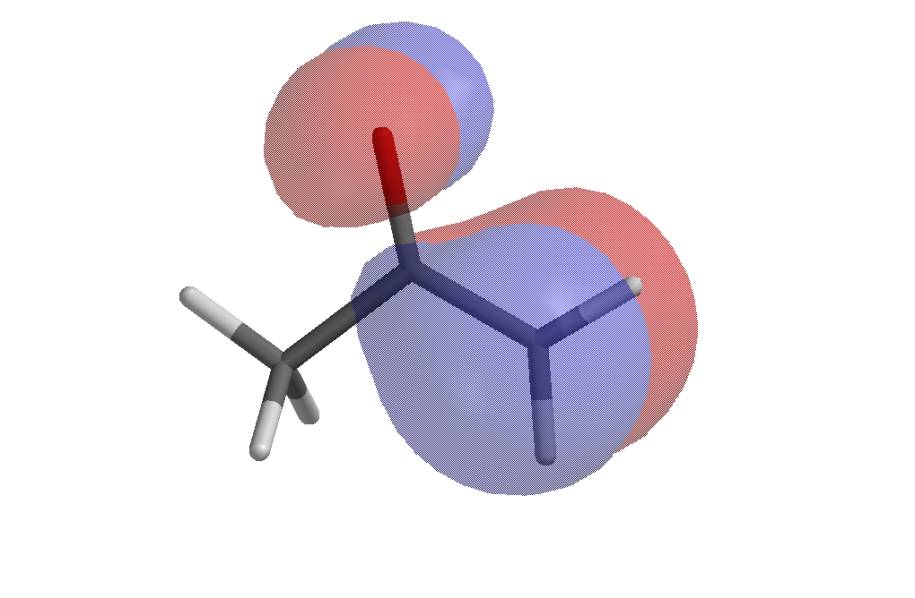
The HOMO of acetone enolate shows the carbon to be a more likely nucleophile than the oxygen (the LUMO of an electrophile would overlap better with the HOMO at the carbon than at the oxygen).