Example exam questions
1. Answer part
(a) and either part (b) or part (c).
(a) The statements below relate to the Hückel
molecular orbital method, and the approximations made in it. Assess each of the following statements as
either true or false.
In Hückel Theory:
Only
p electrons are considered (1
mark)
Each
molecular orbital is made up of a linear combination of atomic orbitals (1 mark)
The
overlap integrals for orbitals on adjacent atoms are given the value b. (1
mark)
All
Coulomb integrals (Hrr)
are given the value a
(1
mark)
The
Coulomb integral, a, and the resonance integral, b, are positive energy quantities. (1
mark)
The
value of the resonance integral for adjacent carbon atoms is zero. (1
mark)
The
value of the resonance integral for non-adjacent carbon atoms is b. (1
mark)
In
finding the molecular orbitals, the secular determinant must be zero for a
non-trivial solution. (1
mark)
(b) (i)
Consideration of orbital symmetry can often be useful in predicting
whether cycloaddition reactions will proceed thermally. Briefly describe the process which would be
applied in such an analysis of a reaction.
Identify the role of ‘correlation diagrams’. It is not necessary to give a detailed example. (5 marks)
(ii) Use the principles of orbital symmetry
conservation to predict whether or not the cycloaddition reaction of two ethene
molecules to form cyclobutane is allowed thermally. Illustrate the answer clearly, indicating the relevant symmetry
elements. Without giving a full
description, indicate briefly how the analysis would differ for the photochemical
reaction. (11
marks)
(c) Consider
trimethylenemethane:
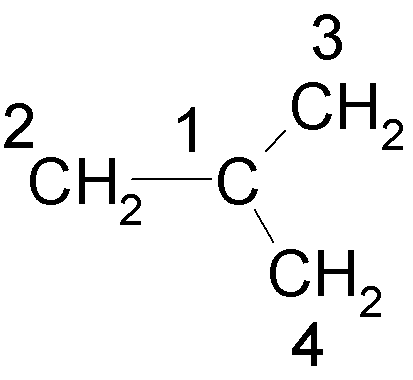
(i) Write down
the Hückel secular determinant for this molecule, using the numbering scheme
shown, and the convenient substitution x
= (a – E)/b. (2 marks)
(ii) The secular
determinant for trimethylenemethane can be expanded as
y2 – 3y = 0
where y = x2 (x
= (a – E)/b).
Find the energies
of the molecular orbitals. Draw a
molecular orbital diagram showing the energies of the molecular orbitals, and
how the four p electrons occupy these orbitals. Comment on the electronic structure of this
compound.
(10
marks)
(iii) Calculate
the delocalization energy of trimethylenemethane. Butadiene (CH2-CH-CH-CH2)and trimethylenemethane
are structural isomers. The
delocalization energy of butadiene is found to be 0.472b. Which compound is
expected to be more stable, and why? (4 marks)
2. Answer 3 of
parts (a-d). All parts carry equal marks.
(a) Write down
the Hückel secular determinant for 1,3-butadiene (CH2CHCHCH2),
using the convenient substitution x = (a – E)/b.
The determinant
can be solved as
y2 – 3y +1 =
0
where y = x2. Calculate the energies of the Hückel molecular orbitals of
1,3-butadiene, in terms of a and b. Also calculate
the delocalization energy of 1,3-butadiene (hint: the energy of the lowest
Hückel molecular orbital in ethene is a + b)
(b) The thermal
ring opening of cyclobutene (C4H6) to give 1,3-butadiene
(CH2CHCHCH2) occurs in a conrotatory fashion. Illustrate
what is meant by the terms conrotatory and disrotatory for this
reaction. Indicate clearly separate
symmetry elements for the conrotatory and disrotatory paths that could be used
to analyse and explain the observed behaviour. State the symmetry of the lowest
energy molecular orbital (only) of 1,3-butadiene, and of the lowest energy
molecular orbital corresponding to the breaking s bond in
cyclobutene, with respect to each of these symmetry elements.
(c) The occupied
Hückel p molecular orbitals of 1,3-butadiene (CH2CHCHCH2)
can be written as:
ya = 0.371f1 + 0.602f2 + 0.602f3 + 0.371f4
yb = 0.602f1 + 0.371f2 – 0.371f3 – 0.602f4
Using the Hückel
approximations, show that ya is normalized. Sketch both ya and yb. State, with a reason, which
orbital is lower in energy. Calculate the Hückel p bond order
between the central carbon atoms (atoms 2 and 3) and between two other adjacent
atoms (e.g. atoms 1 and 2). Comment on the values obtained in relation to a
simple drawing of the structure of 1,3-butadiene.
(d) Draw the
frontier orbitals involved in (i) the dimerization of ethene and (ii) the
Diels-Alder reaction of 1,3-butadiene with ethene. By considering their
interactions, show which of these reactions is allowed and which is forbidden.
For ethene dimerization, show similarly how photochemical excitation can be
used to change the situation.
Determinants
For this course,
you need to be able to calculate determinants. A determinant is written like a matrix, but with straight lines
down each side instead of brackets. For a two by two matrix,
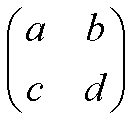
the determinant is:
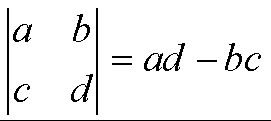
For example:
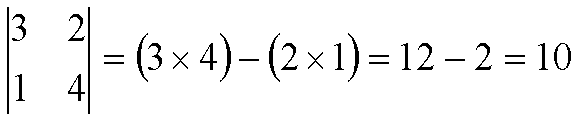
To solve bigger
determinants, you need to break them down into two by two determinants. For a three by three determinant:
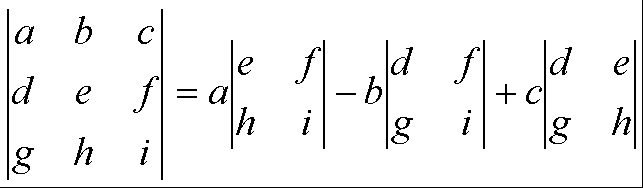
Notice what is
happening here: the numbers along the top row are ‘taken out’, one at a time,
and each multiplies a smaller (two by two) determinant (made from the numbers
remaining when the row and column containing the number taken out are
excluded). For the first number (a),
the sign of this term is positive, for the second (b), it is negative,
for the 3rd it is positive, and so on, alternating positive and
negative. For example:
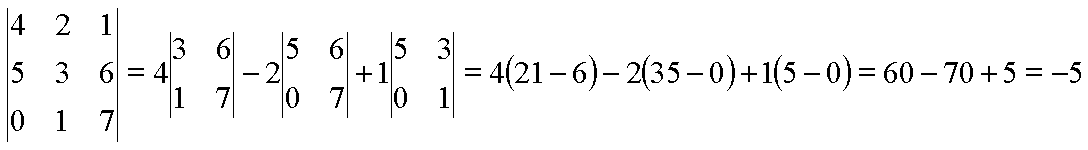
The biggest
determinant you would need to solve is a four by four determinant (see the
example of the secular determinant of 1,3-butadiene in your lecture
notes).