Chapter 6
The influence of surface preparation on the electrochemistry of boron
doped diamond
6.1. Introduction
A range of
redox processes has been studied at highly boron doped diamond electrodes. It has been reported that at such diamond
electrodes those inorganic redox couples that are oxidised and reduced via an
outer sphere electron transfer mechanism show reversible electrochemical
behaviour 1. Electrochemical impedance spectroscopy of diamond
electrodes immersed in solutions containing reversible redox couples indicates
that the mechanism of electron transfer is dependent on the surface termination
2. The redox
electrochemistry at highly boron doped diamond electrodes has been investigated
for a range of organic species in aqueous solution. For a number of couples oxidation and reduction have been
observed within the available potential window, these include phenol 3, pyridine 4, dopamine 5 and anthraquinonedisulfonate
6. Studies in which the surface
termination of the diamond electrodes has been considered indicate that the
overpotential for the oxidation of ascorbic acid increases when the surface is
oxygenated 5. However, the
majority of studies of organic oxidation have focused on electrodes biased at a
positive potential that lies in the water breakdown region. Investigations have shown that remediation
of polluted water may be achieved using a positively biased diamond electrode 7. It has been
suggested that the organic species are oxidised by OH radicals which are
generated on the oxidation of water 7. For example,
Iniesta et al. 3 have reported that when low overpotentials are applied the electrochemical oxidation of phenol at synthetic boron doped diamond
electrodes in acidic media occurs via direct electron transfer and results in a
polymeric film on the surface. Whilst,
during electrolyses in the potential region of water decomposition indirect
oxidation reactions take place which, depending on the applied current and
phenol concentration, may result in the complete combustion of phenol to CO2
or the partial oxidation of phenol to other aromatic compounds.
Studies of boron doped
diamond electrodes in non-aqueous solvent are more limited. Fujishima et al. reported that diamond electrodes in non-aqueous solvent
posses an increased potential window, allowing a fifth peak for the reduction
of C60 to be observed 8. In addition diamond electrodes have been used in the
generation of solvated electrons in ammonia 9. This chapter is concerned with electrochemistry of
boron doped diamond electrodes immersed in acetonitrile. Specifically, boron doped diamond electrodes
are employed in a study of the cyclic voltammetry of benzoquinone
reduction. The importance of diamond
electrode surface preparation in determining the mechanism of reduction is
described.
6.2. Experimental set-up
Electrochemical experiments were performed using a three electrode
system. This section descibes the apparatus used.
6.2.1. Electrolyte
solutions
Anhydrous acetronitrile (99.9% pure) was used as solvent and 0.1 mol
dm-3 tetrabutylammonium perchorate (TBAP) as supporting electrolyte.
Different solutions were prepared contaning:
·
1´10-3
mol dm-3 of ferrocene (FeCp2) in 0.1 mol dm-3 TBAP in MeCN.
·
1´10-3
mol dm-3 of 1,4-benzoquinone
in 0.1 mol dm-3 TBAP in MeCN
·
1 mol dm-3 of KCl in deionised water
(18.3 MW cm ultrapure deionised
water (Millipore)).
Glassware was cleaned with a five step process (cross reference
section 5.3.1) and it was kept in the dry box under argon atmosphere.
All the solutions were prepared under dry box conditions to ensure
that no moisture or oxygen was added to the solutions. To double check that no
residual oxygen was present in the solutions they were purged with standard
laboratoy grade oxygen-free argon (Ar) for aproximmately 10 minutes inside the
dry box before any electrochemical experiment was done.
6.2.2. Growing characteristics of the
samples
The boron concentration
employed corresponded to a dopant density of 1021 cm-3,
i.e., the samples were degenerately doped. Diamond deposition was performed for
six hours, giving a film thickness of approximately 5 mm. The film was cooled in the chamber under a
hydrogen atmosphere. Electrical
contacts were silver paint contacts (see chapter 3 for further details). The quality of the diamond films was
assessed using Raman spectroscopy and scanning electron microscopy, sem (see chapter 2 for further details).
6.2.3. Surface sample preparation
Prior to the electrochemical studies the as-prepared hydrogen terminated samples were stored under vacuum. Oxygen terminated surfaces were prepared by immersing the diamond electrodes in a hot cromic acid solution (potassium dichromate satured (K2Cr2O7) in hot sulphuric acid (H2SO4)) 10. Regeneration of a hydrogenated surface was achieved by placing the electrodes in aqueous 1 mol dm-3 KCl solution and applying a potential in the hydrogen evolution region for six hours. The sample was then dried for one hour in an oven at 100 ºC and then stored under vacuum.
6.2.4. Electrochemical Cells
The main type of cell used
in these studies was described in section 5.3.2.
6.2.5. Counter Electrodes
The counter electrodes have
been described in section 5.3.3.
6.2.6. Reference Electrodes
The reference electrodes
have been describes in section 5.3.4.
6.2.7. Glassy Carbon Electrodes
4 mm in diameter (2 mm
thick) glassy carbon disks were mounted in 5 cm length glass tubes. To reach
the electrical contact a copper wire was used sealed and protected using
Araldite resin. The glass tube was sealed in the top by Araldite resin (see figure
6.1).
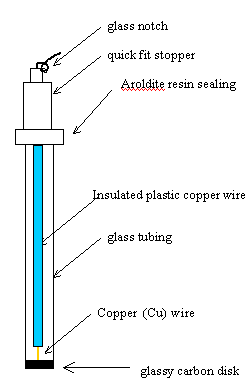
Figure 6.1. A schematic diagram of a glassy carbon electrode.
6.2.8. Potentiostats
Cyclic voltammograms
measurements were performing using EG&G Princeton Applied Research
Potentiostat/Galvanostat Model 273; controlled using Research Electrochemistry
software (version 4.3).
6.2.9. The dry box
The dry box used in these
studies have been described in section 5.3.6. All electrochemical experiments
(except hydrogen regeneration) were performed in the dry box in such conditions
that the amount of water vapour was less than 5 ppm by volume.
6.3. Cyclic voltammograms
At the beginning of the
experiment a cyclic voltammogram was recorded using a glassy carbon working
electrode. A typical i-E curve for a glassy carbon electrode
immersed in the 1.0´10‑3 mol
dm-3 benzoquinone / 0.1 mol dm-3 TBAP / acetonitrile
solution is shown in figure 6.2.
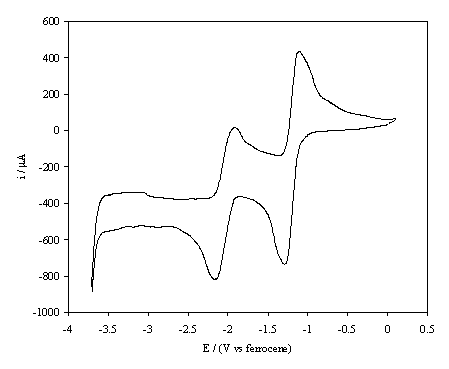
Figure 6.2. A cyclic voltammogram recorded at a glassy
carbon electrode immersed in 1´10-3 mol dm-3 1,4-benzoquinone/0.1 mol dm-3
TBAP in anhydrous acetronitrile. The i-E
curve was recorded at scan rate of 0.1 V s-1.
The curve shows two reversible reduction peaks the first at -1.40 V and the second at -2.29 V, these correspond to the formation of Q¯• and Q2- respectively, where Q represents 1,4-benzoquinone. The behaviour observed is typical for the reduction of benzoquinone in an aprotic solvent 11, 12 and confirms that the electrolyte employed in the experiments was dry. Cyclic voltammograms for a glassy carbon working electrode were taken periodically during the studies and in all cases curves similar to that in figure 6.2 were observed. This indicates that at no stage, even following ex-situ surface processing, was water introduced into the electrolyte.
The i-E curve for the reduction of benzoquinone at an oxygenated boron
doped electrode is displayed in figure 6.3.
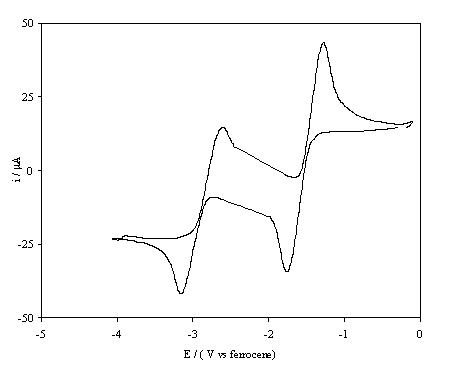
Figure 6.3. A cyclic voltammogram recorded at an
oxygen terminated, high boron doped, diamond electrode immersed in 1´10-3 mol dm-3
1,4-benzoquinone/0.1 mol dm-3 TBAP in anhydrous acetronitrile. The i-E curve was recorded at scan rate of
0.1 V s-1.
The plot resembles that at a
glassy carbon electrode except that the peaks occur at lower potentials, this
is due to the fact that iR compensation
was not employed when recording the data.
The voltammogram implies that benzoquinone undergoes two reversible
one-electron reductions at an oxygenated boron doped diamond surface. In contrast, the benzoquinone reduction at
the as-prepared boron doped diamond surface differs markedly from that at a
glassy carbon electrode.
The cyclic voltammogram for the reduction of benzoquinone at an as-prepared electrode is shown in figure 6.4.
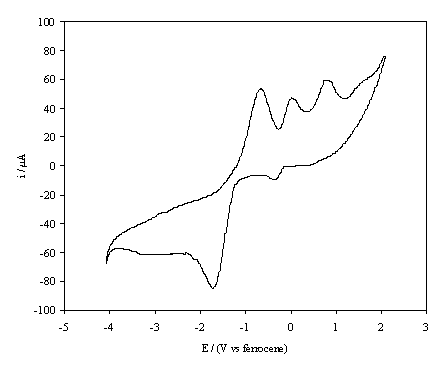
Figure 6.4. A cyclic voltammogram recorded at an
as-grown, high boron doped, diamond electrode immersed in 1´10-3 mol dm-3
1,4-benzoquinone/0.1 mol dm-3 TBAP in anhydrous acetronitrile. The i-E curve was recorded at scan rate of
0.1 V s-1.
Similar cyclic
voltammograms, see figure 6.5, were found for the initial cycles of
benzoquinone reduction at boron doped electrodes at which, following chromic
acid treatment, hydrogen had been generated in aqueous solution. However, it is of note that following a
period of one hour of continual cycling the i-E
curve at the re-hydrogenated electrode surface began to resemble that of
the oxygenated surface. The cyclic
voltammograms displayed in figures 6.4 and 6.5 are very similar to those
reported for benzoquinone reduction in the presence of a weak acid 13, 14.
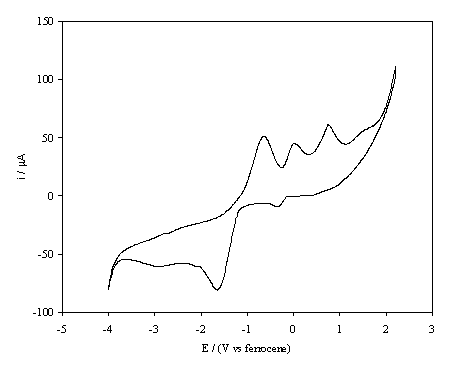
Figure 6.5. A cyclic voltammogram recorded at re-hydrogenated, high boron doped, diamond electrode immersed in 1´10-3 mol dm-3 1,4-benzoquinone/0.1 mol dm-3 TBAP in anhydrous acetronitrile. The i-E curve was recorded at scan rate of 0.1 V s-1.
This suggests that the small oxidation peak at the start of the scan, at a potential of -0.2 V corresponds to the reduction of protonated quinone (the scan depicted is not the first of the series) whilst, the peak at -1.73 V is due to the ECE process,
Q + e ® Q¯• + H+ ® QH• + e ® QH¯,
which may be accompanied by the disproportionation reaction,
Q + QH¯ ® Q¯• + QH•.
The shoulder at more negative potentials appears to result from the irreversible reduction of Q¯•. In the cyclic voltammetry of 1,4-benzoquinone in the presence of a weak the three oxidation peaks are assigned as the oxidation of Q¯• (-0.55 V), QH¯ (0.00 V) and QH2 (+0.75 V) respectively.
The observation that the redox behaviour of benzoquinone at the hydrogenated boron doped diamond surface resembles that normally observed only in the presence of a weak acid is surprising. As mentioned above, cyclic voltammetry was regularly performed at a glassy carbon electrode in order to assess if the solution remained dry during the experiments. For these control experiments no evidence of a weak acid in the solution was found, suggesting that the hydrogenated diamond surface is responsible for protonation of the Q¯• radical and not a solution phase species. This inference is supported by the observation that in a common solution the reaction at the oxygenated surface is markedly different to that at the hydrogenated surface. It has been established 15 that there is a sub-surface layer of hydrogen at as-grown chemical vapour deposition, cvd, diamond that may be responsible for the high surface conductivity of the material. Treatment of the diamond with oxidising acids results in an oxygenated surface and a loss of hydrogen from the sub-surface region, resulting in a lowering of the surface conductivity 16. The sub-surface hydrogen in diamond is restored when hydrogen gas is electrochemically generated at the electrode/electrolyte interface. It appears that the hydrogen in the sub-surface may act as a source of protons in electrochemical reactions.
6.4. Conclusions
It has been demonstrated that benzoquinone can be reduced and oxidised at boron doped diamond electrodes in non-aqueous solvents. The mechanism of reduction is dependent on the preparation of the diamond surface. At a surface treated with hot chromic acid, oxygen terminated, the electrochemistry resembles that at glassy carbon, i.e., two reversible reduction processes are observed. In contrast, at an as-prepared electrode or one at which hydrogen has been generated, hydrogen terminated surface, the electrochemistry indicates the presence of a source of protons. Rigorous tests showed that the electrolyte was not contaminated and suggest that hydrogen in the diamond sub-surface may participate in electrochemical processes.
6.5. References
1 S. Alehashem, F. Chambers,
J. W. Strojek, G. M. Swain, and R. Ramesham, Anal. Chem., 1995, 67,
2812.
2 M. N. Latto, D. J. Riley,
and P. W. May, Diam. Relat. Mater.,
2000, 9, 1181.
3 J. Iniesta, P. A. Michaud,
M. Panizza, G. Cerisola, A. Aldaz, and C. Comninellis, Electrochim. Acta, 2001, 46,
3573.
4 J. Iniesta, P. A. Michaud,
M. Panizza, and C. Comninellis, Electrochem.
Commun., 2001, 3, 346.
5 H. Notsu, I. Yagi, T.
Tatsuma, D. A. Tryk, and A. Fujishima, Electrochem.
Solid State Lett., 1999, 2, 522.
6 J. Xu, Q. Chen, and G. M.
Swain, Anal.Chem., 1998, 70, 3146.
7 G. Fóti, D. Gandini, C.
Comninellis, A. Perret, and W. Haenni, Electrochem.
Solid State Lett., 1999, 2, 228.
8 Z. Y. Wu, T. Yano, D. A.
Tryk, K. Hashimoto, and A. Fujishima, Chem.
Lett., 1998, 503.
9 F. J. Del Campo, C. H.
Goeting, D. Morris, J. S. Foord, A. Neudeck, R. G. Compton, and F. Marken, Electrochem. Solid State Lett., 2000, 3, 224.
10 G. Pastor-Moreno and D. J.
Riley, Electrochim. Acta, 2002, 47, 2589.
11 D. H. Evans, 'Encyclopedia
of Electrochemistry of the Elements', ed. A. J. Bard, M. Dekker Inc., 1978.
12 J. M. Hale and R. Parsons, T. Fad. Soc., 1963, 59, 1429.
13 A. J. Bard and L. R.
Faulkner, 'Electrochemical Methods Fundamentals and Applications', 2001.
14 B. R. Eggins and J. Q.
Chambers, J. Electrochem. Soc., 1970,
117, 186.
15 H. J. Looi, L. Y. S. Pang,
A. B. Molloy, F. Jones, J. S. Foord, and R. B. Jackman, Diam. Relat. Mater., 1998, 7,
550.
16 K. Hayashi, S. Yamanaka, H.
Watanabe, T. Sekiguchi, H. Okushi, and K. Kajimura, J. Appl. Phys., 1997, 81,
744.