 Jeroen van Duijneveldt's research group
Jeroen van Duijneveldt's research group
 Soft condensed matter
Soft condensed matter
The term soft condensed matter refers to a range of systems that fall between simple liquids and solids, for instance colloidal suspensions,
emulsions, liquid crystals, and polymers. This includes many systems of practical or biological importance, such as inks, paints, shampoos,
foodstuffs, milk and blood. Real systems tend to be complex, consisting of many components that are often difficult to characterise in detail.
Well-defined model systems are therefore studied instead. A central theme is the use of polymers to control particle interactions,
structure and phase behaviour in colloidal suspensions.
Please contact me if you are interested in studying for a Ph.D. in any of the areas listed
 Colloidal liquid crystals
Colloidal liquid crystals
Particles that are sufficiently non-spherical (either rod-like or plate-like) can form liquid crystalline phases, in particular a
nematic phase, on increasing concentration. For the first time, we have succeeded in obtaining such ordered suspensions starting from
a natural clay, sepiolite. A pronounced fractionation with respect to rod length is found, with the long rods accumulating in the nematic
phase.
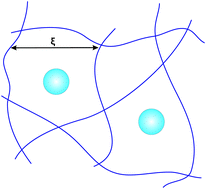 Attraction through repulsion
Attraction through repulsion
Presence of non-adsorbing polymer in solution induces a depletion attraction between suspended particles. This can lead to phase separation, aggregation
or gelation. This mechanism is relevant to practical formulations and addition of polymer allows a fine control of particle attractions.
Currently, we are studying this effect in the so-called protein limit, where the polymer chains are much larger than the suspended
(nano-)particles. Addition of polymers is useful to help proteins crystallise.
- G.A. Vliegenthart, J.S. van Duijneveldt and B. Vincent, Phase transitions and gelation of silica-polystyrene mixtures in benzene, Faraday Discuss. 123:65-74, 2003.
- Y. Hennequin, M. Pollard and J.S. van Duijneveldt, Phase behavior of binary mixtures of sterically stabilized colloids with large size asymmetry, J. Chem. Phys. 120(2):1097-1104, 2004.
- A.I. Campbell, V.J. Anderson, J.S. van Duijneveldt, P. Bartlett, Dynamical Arrest in Attractive Colloids: The Effect of Long-Range Repulsion, Phys. Rev. Lett., 94: 208301, 2005.
- Z. X. Zhang and J.S. van Duijneveldt, Experimental Phase Diagram of a Model Colloid-Polymer Mixture in the Protein Limit, Langmuir , 22: 63-66, 2006.
- K.J. Mutch, J.S. van Duijneveldt, and J. Eastoe, Colloid-protein mixtures in the protein limit, Soft Matter 3: 155-167, 2007.
Unbinding books
Polymers and surfactants are used to prepare non-aqueous suspensions of clay platelets, in which the original clay
stacks are fully exfoliated into single sheets. High aspect ratio platelets can be obtained this way.
Small-angle X-ray scattering is a powerful technique to study these systems
at the nanometre lenght scale and is done in collaboration with Prof.
Robert Richardson.
- S.D. Zhang, P.A. Reynolds and J.S. van Duijneveldt, Phase separation in mixtures of colloidal platelets and non-adsorbing polymer: a scaled particle treatment, Mol. Phys. 100(18):3041-3048, 2002.
- S.D. Zhang, P.A. Reynolds and J.S. van Duijneveldt, Phase behavior of mixtures of colloidal platelets and nonadsorbing polymers, J. Chem. Phys. 117(21):9947-9958, 2002.
- E.S.H. Leach, A. Hopkinson, K. Franklin and J.S. van Duijneveldt, Non-aqueous suspensions of Laponite and montmorillonite, Langmuir, 21: 3821-3830, 2005.
- J. Connolly, J.S. van Duijneveldt, S. Klein, C. Pizzey, and R.M. Richardson, Effect of surfactant and solvent properties on the stacking behaviour of non-aqueous suspensions of organically modified clays, Langmuir 22: 6531-6538, 2006.
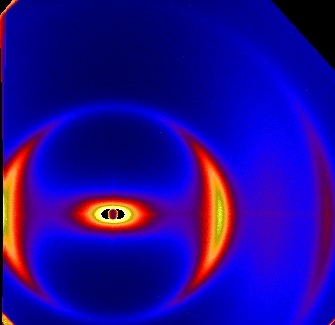 Liquid crystals as structured solvents
Liquid crystals as structured solvents
Liquid crystal suspensions consist of a (thermotropic) liquid crystal used as solvent for colloidal particles. In the nematic phase the presence
of the colloidal particles (spheres, rods, platelets) introduces defects in
the liquid crystalline host material which leads to novel particle interactions.
The X-ray scattering pattern on the left is from a clay suspension in 5CB. The nematic liquid crystal is
aligned in a magnetic field and gives rise to a diffuse scattering peak. The bright scattering peaks are due to the suspended clay platelets and
demonstrate that the platelets here are aligned perpendicular to the liquid crystal director.
- C. Pizzey, S. Klein, E. Leach, J.S. van Duijneveldt, and R.M. Richardson, Suspensions of colloidal plates in a nematic liquid crystal: a small angle X-ray scattering study, J. Phys.: Condens. Matter 16 (15): 2479-2495, 2004.
- J.S. van Duijneveldt, S. Klein, E. Leach, C. Pizzey, R.M. Richardson, Large scale structures in liquid crystal / clay colloids. J. Phys.: Condens. Matter, 17: 2255-2267, 2005.
 Models
for liquid crystals
Models
for liquid crystals
Molecules or particles that are strongly non-spherical,
that is either rod-like or disc-like, tend to form liquid crystalline phases
that display a degree of order in between an isotropic liquid and a crystal.
We use Monte Carlo computer simulations to study how properties of the
molecules, such as their shape, flexibility and the presence of polar groups
influences the structure of the liquid crystalline phases and the phase
behaviour. This work is done in collaboration with
Prof. Mike Allen (Warwick), Prof. George Jackson (Imperial College) and Dr. Alejandro Gil-Villegas (Leon).
- J.S. van Duijneveldt and M. P. Allen. Free energy barriers for interlayer diffusion in the smectic-A phase of hard spherocylinders. Mol. Phys. 90(2):243-250, 1997.
- J.S. van Duijneveldt and M.P. Allen, Computer simulation study of a flexible-rigid-flexible model for liquid crystals, Mol. Phys. 92(5):855-870, 1997.
- J.S. van Duijneveldt, A. Gil-Villegas, G. Jackson, and M.P. Allen. Simulation study of the phase behavior of a primitive model for thermotropic liquid crystals: Rodlike molecules with terminal dipoles and flexible tails. J. Chem. Phys. 112(20):9092-9104, 2000.
Methods
Projects in our group usually involve the preparation of model systems, such as colloidal spheres or platelets of well-defined size. A variety of characterisation techniques are used, particularly scattering methods (light, X-rays, neutrons) and microscopy. Experimental work is complemented by computer simulations. Much of the work is collaborative and funding comes from a range of sources including industry.People
- Ph.D. student 2011-2015: Phillip Woolston studies colloidal rods and platelets.
- Ph.D. student 2011-2015: Emma Kastrisianaki-Guyton studies nanomaterials, in collaboration with Terence Cosgrove.
- Ph.D. student 2009-2013: Cathy Cooper studies polymer adsorption on particles, in collaboration with Terence Cosgrove and Stuart Prescott.
- Ph.D. student 2009-2013: Katie Bayliss studies colloidal mixtures.
- Ph.D. student 2008-2011: Yannan Cui studies suspensions of clay platelets
Past group members
- Ph.D. student 2007-2010: Juan Zhou studied colloid - polymer mixtures, in collaboration with Brian Vincent
- Ph.D. student 2007-2011: Nisha Doshi studied suspensions of clay platelets, in collaboration with Terence Cosgrove.
- EU Marie Curie Fellow 2008-2010: Dr Giorgio Cinacchi studied phase transitions in colloidal rods and platelets (publication list)
- Ph.D. student 2005-2008: Yan Zhang studied clay-polymer composites, in collaboration with Terence Cosgrove.
- Ph.D. student 2006-2009: Nuttawisit Yasarawan studied suspensions of rod-like clay particles
- Ph.D. student 2005-2008: Kevin Mutch studied microemulsion - polymer mixtures, in collaboration with Julian Eastoe.
- Ph.D. student 2005-2008: Tim Kelly studied the use of polymers to control suspension flow and sedimentation.
- Postdoctoral fellow 2005-2007: Aaron Olsen studied dynamical arrest in colloidal model systems in collaboration with Professor Brian Vincent, as part of a EU Marie Curie RT Network
- Ph.D. student 2003-2006: Zexin Zhang studied colloidal spheres, rods and platelets. Currently at the University of Pennsylvania.
- Visiting research fellow 2001-2004: Susanne Klein is based at Hewlett Packard laboratories.
- Ph.D. student 2001-2004: Claire Pizzey studied optical properties of novel colloid - liquid crystal composites. Currently at the University of Wisconsin - Madison
- Ph.D. student 2001-2004: James Tait studied interactions between colloidal particles suspended in liquid crystals, in collaboration with Paul Bartlett.
- Postdoctoral research assistant 2003-2004: Andrew Campbell studied colloidal crystallisation using confocal microscopy, in collaboration with Paul Bartlett. Currently at the University of Utrecht
- Ph.D. student 2000-2003: Yves Hennequin studied mixtures of sterically stabilised colloidal particles. Currently at the University of Amsterdam
- Ph.D. student 1999-2002: Paul Seymour studied the behaviour of spherical particles with a thick stabilising polymer layer. Currently at Diageo.
- Ph.D. student 1999-2002: Edward Leach studied non-aqueous clay suspensions. Currently at Sun Chemical.
- Postdoctoral research assistant 2001-2002: Valerie Anderson studied colloidal crystallisation using confocal microscopy, in collaboration with Paul Bartlett. Currently at Schlumberger.
- Postdoctoral research assistant 2000-2002: Shu-Dong Zhang studied the phase behaviour of mixtures plate-like particles and polymers, in collaboration with Dr Paul Reynolds. Currently at Leicester.
- Postdoctoral fellow 1999-2001: Gerrit Vliegenthart studied gelation and phase separation of spherical particles, in collaboration with Professor Brian Vincent and Professor Bob Evans. Currently at the Institute for Solid State Research in Juelich (Germany)
- Ph.D. student 1996-1999: James Weeks (main advisor Professor Brian Vincent) studied aggregation of sterically stabilised colloids.