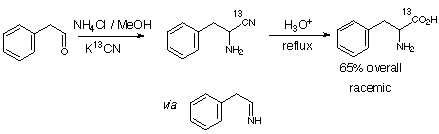
The Strecker Synthesis.

This diagram was copied from the home page of Dr. R. J. Cox.
The product of the Strecker Synthesis is a racemic mixture of phenylalanine, which is one of the components of Aspartame.
The racemic mixture is composed of both L and D phenylalanine. Only L phenylalanine is used to make Aspartame. Therefore, the two forms must be separated. This can be done by reacting the racemic mixture with Ac2O and NaOH. The product of this reaction is then treated with Porcine Kidney Acylase and an organic extraction with H3O+ is then carried out. The L form of phenylalanine will be found in the water layer and the D form will be found in the organic layer.
The acid group on phenylalanine must now be converted into an ester. This is done by treatment of L phenylalanine with MeOH and HCl
Finally, Aspartame can be made by reaction of the compound created above with Aspartic acid. The structure of aspartic acid can be found by clicking
here.
The amine group on Aspartic acid must be protected with Cbz and the acid group nearest the amine must be protected with benzyl as they are a reactive sites that we do not wish to react with the pheylalanine. The other acid group must then be activated with BOP. BOP is removed as phenlyalanine is added, but the protecting groups remain. Cbz is removed by a reaction of H2/PtO2 with MeOH and CHCl3 and benzyl is removed by reation of H2/Pd/C and MeOH and CHCl3. Aspartame has now been made.