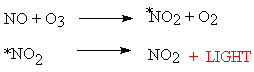
Chemiluminescence is the production of light from a chemical reaction. In fireflies nearly 100% of energy produced in the reaction is emitted as light whereas a light bulb produces only a small fraction of its energy as light and therefore is much less efficient than luminescence in nature.
There are many chemical reactions which demonstrate luminescence as a means of energy release as opposed to heat. A common chemiluminescent reaction is a flame. The reaction between a fuel and an oxidant produce excited state products that emit light but this is not a good example of a chemiluminescent reaction. This is because incandescent particles are often present. These are particles which simply glow because they are at such a high temperature. The amount of heat in the flame makes these particles prevalent and therefore most of the light produced by the flame is from incandescent particles rather than luminescence.
Another example is the reaction between nitrogen monoxide and ozone;
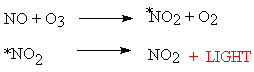
Excited state NO2 is formed and little of the energy produced is released as heat but as light and therefore the products do not incandesce to any significant degree.
Bioluminescence and chemiluminescence are not the same as fluorescence or phosphorescence as may often be believed.