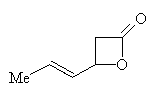
It was first made by the hydrolysis of oil distilled from unripe mountain-ash berries in 1859. In 1900 the first synthesis was performed by Doebner. Sorbic acid was made from crotonaldehyde and malonic acid in pyridine. The yield was improved with use of malonic acid salts. One of the first commercial methods involves the reaction of ketene with crotonaldehyde in presence of BF3 in ether at 0°C. This lactone below is formed which then reacts with acid to give a 70% yield.

Nowadays, a few modifications have been made which include the use of catalysts such as zinc, cadmium, metal oxide, etc. These produce a condensation adduct. After removing the unreacted components and solvent the adduct is decomposed by in acidic media or pyrolysis. Proper operation of acidic decomposition can give high yields of the trans,trans isomer of sorbic acid, whereas the pyrolysis gives a mixture of isomers. The isomeric mixture can be converted to the thermodynamically more stable trans,trans form in the presence of iodine, alkali or sulfuric or hydrochloric acid.