School
of
Chemistry
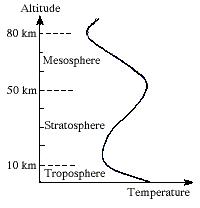
Production and DestructionIn the stratosphere most of the oxygen exists as O2 rather than atomic oxygen. Because of these relative concentrations the most likely fate of any oxygen atoms produced by photochemical decomposition of O2 is their subsequent collision with diatomic oxygen molecules. This results in the production of ozone, and is the source of all the ozone in the stratosphere. The formation of ozone requires UV-C photons from sunlight, and during daylight ozone is constantly being formed by this process. The heat generated by this reaction in the stratosphere leads to a temperature inversion in the atmosphere, where the air at a given altitude is warmer than that below it.
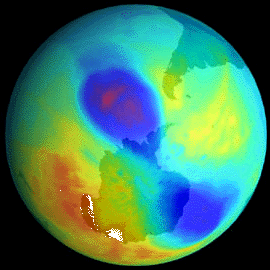
(image taken from www.nobel.se/chemistry/laureates/1995/press.html) The characteristic of ozone that makes it so valuable, it's abilits to absorb ultraviolet radiation, also leads to it's destruction. Ozone efficiently absorbs UV light with wavelengths shorter than 320nm, and the excited state produced by this absorption undergoes a dissociation reaction. The oxygen atom and O2 molecule produced by this dissociation may be in a number of different electronically excited states. Ozone can also be destroyed in a catalytic process, either involving 'natural' or manufactured species, designated as X. These react efficiently with ozone by removing an oxygen atom from it, producing XO. These XO molecules then react with oxygen atoms to produce O2 and to reform X. The catalyst species, X, may be 'natural' (one which would occur in an unpolluted atmosphere), such as NO., or species from manufactured compounds. Cl. released from CFCs and Br. released from halons are two of the most important chemicals associated with ozone depletion. |

 |
 |
 |
 |
 |
 |