Emulsifiers are surface active molecules (surfactants), which lie at the water-fat interface since each molecule of an emulsifier contains a hydrophilic (water loving) portion and a lypophilic (oil loving) portion. They decrease the energy it takes for the fat globules to coalesce as they reduce the interfacial tension of the globules by disrupting intermolecular forces at the interface. This promotes destabilization. The fat globules can now partially coalesce (helped by mixing). The fat globules then move to the air interface.
The emulsifiers in ice cream are proteins (the original emulsifier in ice cream was egg yolk!). The ability of the protein to interact with the air and the fat phases depends on its side-chains. Changing the protein thus changing the interactions between the air and the fat phases and therefore properties of the ice cream.
For an example, one common milk protein is B-casein (ref.6), which is very good at sticking to interfaces. Some of the amino acids that frequently occur in B-casein are shown in the table.
| R-group and | Structure | Polar/non-polar |
|---|---|---|
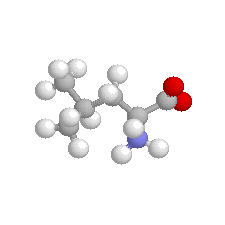
| Leucine |  | non polar |
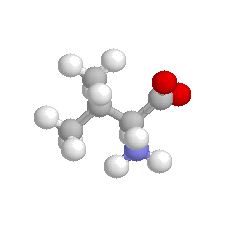
| Valine |  | non polar |
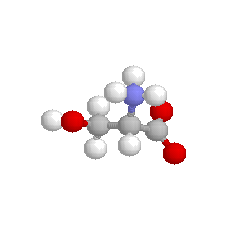
| Serine |  | polar |
The amino acids with polar side chains such as serine and glutamic acid can interact (hydrogen bonding) with water molecules. There are also those that don’t such as leucine, valine and phenylalanine. They have non-polar side chains and so they only interact strongly with non-polar substances such as oil and air (van der Waals interactions).
B-casein has most of the polar residues on one end and the non-polar residues on the other. This is like one giant soap anion whose non-polar ‘tail’ interacts with the air/fat phase and whose head group interacts with the water. The air bubbles may not coalesce due to repulsions of the negatively charged head groups.
The emulsifiers used in ice cream today are mono- and di-glycerides and Polysorbate 80.
The proteins in ice cream can be seen to interact with each phase and shows how complex the structure of ice cream really is.