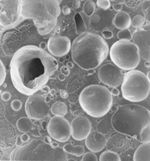
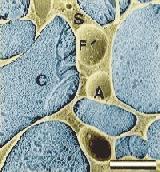
electron micrographs of ice cream ( ref.3/4) a = air bubbles, c = ice crystals, f = fat and s = concentrated aqueous solution
Ice cream is more complex than you might think. It is much more than just ice and cream. Most ice cream is made from water (55%-64%), milk and cream(>28%), sucrose/sugar (10%-14%), and flavourings and additives to help maintain the stability of the frozen structure.
When looking at ice cream at a microscopic level it is found that ice cream is made up of four phases: ice, air, fat and a concentrated aqueous solution. It is the relative amount of these phases and the interactions between them that determines the properties of the ice cream – whether soft and ‘whippy’ or hard.


electron micrographs of ice cream ( ref.3/4) a = air bubbles, c = ice crystals, f = fat and s = concentrated aqueous solution
Ice cream is both a foam and an emulsion. These are examples of colloids as they consist of a dispersion of small particles (<0.5mm) of one phase in another. The air in the ice cream does not mix with the other substances but forms small bubbles in the bulk (a foam). An emulsion also forms as the milk/cream is dispersed in the ice/water as well. The properties of these colloids and therefore the ice cream, depend on the interface that forms between the two phases
Molecules which stick to the interfaces can stabilise the dispersion (small bubbles or fat droplets) and prevent coalescence. These molecules are called emulsifiers. In ice cream the surface of the air bubbles are covered by milk proteins (emulsifiers).
The more air bubbles there are and the smaller they are, the more interface there is to be stabilised. Controlling the protein concentration and type (efficiency as an emulsifier) therefore controls the amount and size of the air bubbles.
The advantage of changing the amount of air in the ice cream is that it alters the amount of ice per ‘mouthful’ and make the ice cream feel warmer.
Another way of controlling the properties of ice cream is to control the amount of ice. As explained earlier, an ice cream contains about 60% water before freezing. An ice cream is eaten far below the freezing point of water and so it could be expected that an ice cream contains 60% ice, however, this is not the case. Not all the water is frozen at the eating temperature (~-20°).
The reason is that the freezing of a liquid is affected by the presence of dissolved molecules (mainly milk proteins and sugars). This depresses the freezing point and is known as a colligative effect. The presence of the solute broadens the range of temperature that freezing will occur as well as when freezing begins. The number of solute molecules determines these effects. Consequently the amount of ice at a given temperature is sensitive to the change in solute concentration. As proteins are fairly large their colligative effect is small in comparison to that of the number of moles of sugar. Therefore, the amount of ice in an ice cream at a given temperature can be estimated by the sugar concentration.
By knowing the amount of ice present the ice cream’s properties can be determined. One property that is largely determined by the amount of ice is how cold the ice cream feels. Why?