8 The Structure Factor
Following Fourier analysis it is found that the scattering amplitude is the sum of complex exponentials. It is well known that the intensity of radiation is proportional to the square of its amplitude, so this could cause some problems if the amplitude is found to have an imaginary part. This turns out to be irrelevant so long as the intensity is real, calculated by multiplying the amplitude by its complex conjugate.
It is found for a crystal of N cells that the amplitude of a Bragg peak is proportional to the 'structure factor'. The structure factor is defined as:
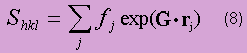
The intensity of a Bragg peak is then proportional to S*S = |S|2. In this expression, the dot product is taken for a specific value of G corresponding to a specific Miller plane. The vector rj represents the position of a general atom in the unit cell relative to a lattice point. The sum is hence taken over all atoms in the unit cell.

