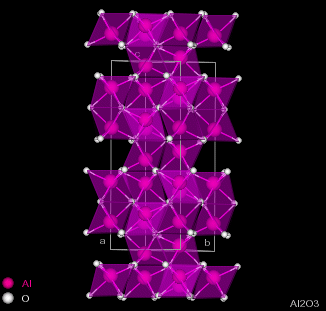
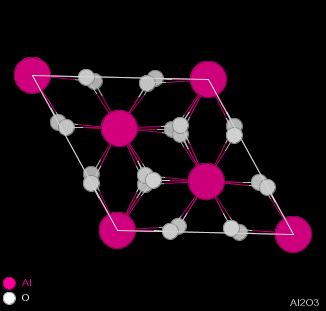
| Figure 2.1 - Structure of Corundum: Side and Top views |
  |
|
image taken from http://jcrystal.com/steffenweber/gallery/StructureTypes/st4.html |
Corundum
| Figure 2.1 - Structure of Corundum: Side and Top views |
  |
|
image taken from http://jcrystal.com/steffenweber/gallery/StructureTypes/st4.html |
Corundum has the chemical formula Al2O3 and consists of hexagonal close-packing oxygen anions, with 2/3 of the octahedral holes filled by aluminium cations. The cations occur two in a row and then skip a site, giving the structure shown in figure 2.1.
This structure gives rise to a number of crystal habits (forms). Click here for a popup showing these crystal habits. For sapphires, a six-sided barrel is common, but other forms exist. These tend to vary depending on where they are found; a result of the environments in which the crystals formed.
Properties
| Colour | variable: white, colourless, blue, red, green, yellow, brown, purple, pink. Also, different colours may be contained in the same crystal (colour zonation) |
| Lustre | vitreous - adamantine |
| Transparency | transparent - translucent |
| Crystal System | trigonal (hexagonal) with 2/3 octahedral holes filled |
| Crystal Habit | varies depending on the type of corundum, and where it is found |
| Cleavage | none |
| Hardness | 9 on Moh's Scale |
| Specific Gravity | 4.0 - 4.1 |
| Streak | white |
Corundum has a hardness of 9 on Moh's Scale, which makes it the second hardest material known. When ground with haematite, magnetite and spinel it is used to make emery, which is a very good abrasive. Although corundum is second only to diamond in hardness, it makes a poor substitute. Diamond is over four times as hard as corundum.
Corundum is also particularly dense for a transparent mineral. Both this and its high hardness can be at least partially attributed to the strong aluminium-oxygen bond strength (511±3kJmol-1).
|
Home ║ Introduction ║ Corundum ║ Colour ║ Synthetic Sapphires ║ Asterias ║ Sapphires of Interest ║ Glossary ║ Links |