The synthesis of tamiflu requires shikimic acid. This acid is extracted
from the pods of star anise.
A 10-step process of complex chemical reactions results in the synthesis
of Tamiflu. Some of the steps use potentially explosive azide chemistry
therefore handling must be carried out with care and relatively mild
conditions must be used.
 |
|
Star anise.
Hoffman La Roche
|
|
|
Not only is the process extremely long, taking approximately 6-8 months to complete, but
30kg of star anise only produces
1kg of shikimic acid. Hence, in order to be able to produce the quantities of Tamiflu required
to fight a bird flu pandemic, either another source of shikimic acid must be found or
another synthesis devised;
Click here to see a diagram of the ten-step synthesis method
|
Roche and its partners are increasingly using fermentation processes to produce
shikimic acid. This involves using a special strain of E.Coli bacteria. When these bacteria
are overfed glucose, or sugar, they produce shikimic acid as a waste product. Scientists
are working to improve the fermentation process, and to increase its efficiency, so that it can
be used to fully meet the shikimic acid requirements of Tamiflu production.
Another synthesis of Tamiflu has been devised by Elias Corey of Harvard University and
colleagues which involves the use of acrylate and butadiene, two readily available and
cheap petrochemicals. A key catalyst used, made from the amino acid proline, favours the
synthesis of the right handed (there is a right and left handed, mirror images, form of this
molecule) form of the drug. Only the right handed form works effectively. Only one of the
steps in the synthesis requires refrigeration, the rest can all be carried out at room
temperature and it produces relatively high yields.
Only the molecule itself is patented, regardless of the synthesis. Hence there are many
research groups attempting to find synthesis which will not require shikimic acid. One is
shown below;
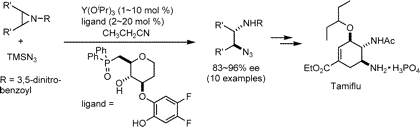 |
|
An alternative mode of synthesis of tamiflu, not utilising shikimic acid.
Click for Weblink
|
The difficulty with devising a suitable synthesis is scaling up a reaction process
from a lab to a commercial scale. Hence the reason a new synthesis without an explosive step
and without a limited reagant, shikimic acid, is desired.
|