![]()
Anethole
The flavour of aniseed and liquorice
![]()
Paul May
University of Bristol
![]()
Molecule of the Month - June 2023
Also available: HTML version.
![]()
Liquorice allsorts - flavoured by anethole.
|
AnetholeThe flavour of aniseed and liquorice
Paul May
Molecule of the Month - June 2023
|
 Liquorice allsorts - flavoured by anethole. |
Yes, it was named by the French chemist Charles Gerhard in 1845 based on the Latin words anethum meaning aniseed and oleum meaning oil.
Yes, and in a number of similar plants, and it is gives them their characteristic smell – and flavour if used in cooking.
Well apart from aniseed and star anise, it’s also found in fennel and liquorice.
 Aniseed (Pimpinella anisum) seeds [Photo: Thamizhpparithi Maari, CC BY-SA 4.0 via Wikimedia Commons] |
 Star anise (Illicium verum) [Photo: Judgefloro, CC0, via Wikimedia Commons] |
 Fennel (Foeniculum vulgare) [Photo: Arnaud 25, Public domain, via Wikimedia Commons] |
 Liquorice (Glycyrrhiza glabra) [Photo: Raffi Kojian, CC BY-SA 3.0 via Wikimedia Commons] |
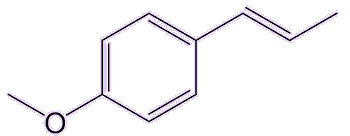
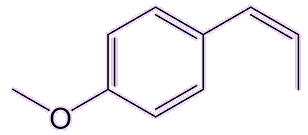
Trans-anethol, the main isomer found in nature, is not toxic in the tiny quantities found in those plants used in foods. It has an isomer (cis-anethol) which is about 20 times more toxic. But luckily that one is only present in trace amounts in those plants.
 |
 |
||
| trans-anethole | cis-anethole |
Yes, that's true, but that was due to another sweet-flavoured chemical present in liquorice root, glycyrrhizin. Ingesting too much liquorice root can lead to symptoms including low potassium levels in the blood, fatigue, and high blood pressure. In some extreme cases it even proved fatal. In many countries, de-glycyrrhizinated licorice (DGL) is now available to avoid the side effects of licorice and is available in capsules, lozenges, wafers and liquid.
Yes, estragole is found in tarragon and basil, and also tastes a bit like aniseed.
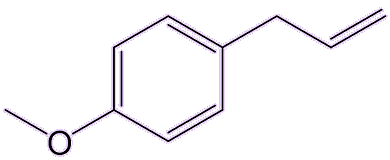 |
|
| Estragole |
And in drinks, too. Because it tastes quite sweet, anethole is often used in spirits to give them a sweet liquorice taste. These include ouzo, raki and absinthe.

Ouzo, Raki and Absinthe
[Photos: Ouzo, AlMare, CC BY-SA 2.5; Raki, Vammpi, CC BY-SA 3.0; Tangopaso, CC BY-SA 3.0, all via Wikimedia Commons]
I know that ouzo behaves strangely when water is added to it. Is that anything to do with anethole?Yes, that’s called the ‘ouzo effect’, which is the spontaneous formation of a microemulsion. Adding water to ouzo or absinthe, which are normally transparent, turns them milky. Why does that happen?Being an oily hydrophobic molecule, anethole is only slightly soluble in water, but it is highly soluble in ethanol, which is present in those aniseed spirits at levels 20-50%. Adding water to the ethanol-water mixture (the spirit) causes the anethole to come out of solution and form oily droplets. Such oil-in-water emulsions are not usually stable because the tiny oil droplets merge together to form much larger droplets which then float to the surface as an oily layer. But in a water-ouzo mixture the rate of coalescence of the droplets is greatly slowed, and they form a stable milky emulsion. Does anethole have any other uses?Because of its distinct taste and smell, it’s added to perfumes, soaps, and toothpastes. It is also used as a flavouring agent in confectionery, baked goods, and other food products. Anethole has been reported to kill some bacteria, yeasts, and fungi, and is also is a promising insecticide, especially against mosquitoes, cockroaches and weevils. It can also work as an insect repellent for mosquitoes and mites, and a derivative of it, anisaldehyde, can even rival the effectiveness of DEET (MOTM Jan 2011). |
 The Ouzo Effect during the preparation of absinthe. As water is added the clear liquid becomes milky. [Photos: Eric Litton, CC BY-SA 2.5 via Wikimedia Commons] |
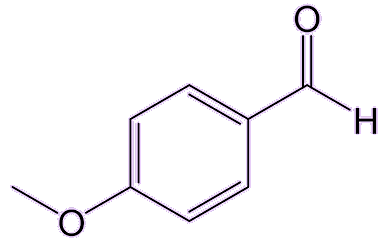 |
|
| Anisaldehyde |
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.22122578]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.22122578]