![]()
Arsenic Pentachloride - AsCl5
(and some other AX5 molecules)
![]()
Simon Cotton
Uppingham School, Rutland, UK
(Now at: University of Birmingham)
![]()
Molecule of the Month January 2003
Also available: JSMol version.
![]()
 |
Arsenic Pentachloride - AsCl5(and some other AX5 molecules)
Simon Cotton
Molecule of the Month January 2003
|
They said it couldn't be made.
PCl5 and SbCl5 were first prepared in the early 19th century, but AsCl5 could not be made, and it was speculated that it was too unstable to exist.
Of course not, otherwise we wouldn't be reading this, but it took a special method to make it.
 How?
How?In 1976, the German chemist Konrad Seppelt (right) found that it could be prepared if a cold mixture of AsCl3 and Cl2 was irradiated with UV light at -100°C,
AsCl3 + Cl2 ![]() AsCl5
AsCl5
It decomposes at temperatures above -60°C.
The UV light splits the chlorine molecule into reactive chlorine atoms, which can combine with the AsCl3 to form molecules of AsCl5 which have not got enough energy to shake themselves apart again.
In his original study, Seppelt examined the vibrational (Raman) spectrum of the reaction mixture at regular intervals. As time went on, the spectrum changed from that of AsCl3 to one characteristic of AsCl5, resembling the known spectra of PCl5 and SbCl5. However, the clincher is that the structure of AsCl5 is now known.
A quarter of a century after his original discovery, Seppelt and his student Silvia Haupt succeded in making yellow crystals of this unstable substance at -125°C by crystallisation from solutions in CHFCl2. X-ray diffraction studies show it has the expected trigonal bipyramidal structure in the solid state. The axial As-Cl distances are 220.7 pm whilst the equatorial As-Cl bonds are 210.6 and 211.9 (averaging 211.45) pm.
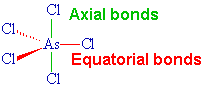 AsCl5 |
 |
No, they aren't. The electron pairs in the "axial" bonds have three 90° repulsions with electron pairs in the "equatorial" bonds, whilst the electron pairs in the equatorial bonds have only two 90° repulsions. It would therefore be predicted that repulsions involving the axial bonds would be stronger and that these bonds would therefore be longer, as is the case.
AsF5 also has a trigonal bipyramidal structure, with As-F (axial) 171.9 pm and As-F (equat orial) 166.8 pm in the gas phase. Molecules of AsF5 are also present in crystals, having As-F (axial) 171.1 pm and As-F (equatorial) 165.6 pm.
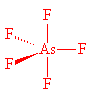 AsF5 |
|
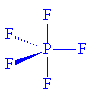 PF5 |
PF5 has a similar structure. Gas phase PF5 molecules have a D3h structure (P-F (axial) 158 pm and P-F (equatorial) 153 pm ; in the solid state at -164°C, P-F (axial) is 158.0 pm and P-F (equatorial) is 152.2 pm.
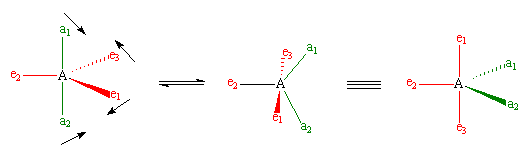
However, when chemists studied the 19F NMR spectrum of PF5, they saw only one signal, even at -100°C, showing there was only one environment for fluorines, which is not what was predicted. It has been suggested that there is interchange of fluorine atoms between the axial and equatorial positions that is rapid on the NMR timescale, the so-called Berry pseudorotation (see image below), which proceeds via a square pyramidal intermediate. In this process, two equatorial bonds (shown in red) move away from each other and become axial bonds at the same time as the axial bonds (green) move together to become equatorial. Animations of this process can be seen in Chime or in Quicktime.
The structure of PCl5 is even more complicated, however.
In the gas phase, it does indeed contain PCl5 molecules, with P-Cl (axial) = 212.4 pm and P-Cl (equatorial) = 201.7 pm. However, crystals of PCl5 are composed of [PCl4]+ and [PCl6]- ions. (P-Cl in the tetrahedral [PCl4]+ ions is 190 pm whilst P-Cl in the octahedral [PCl6]- ions is 211-216 pm).
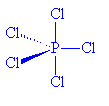 PCl5 |
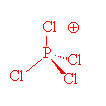 PCl4+ |
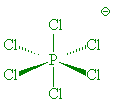 PCl6- |
In addition to that, a metastable solid-state phase is also known that has the structure [PCl4+]2 [PCl6-] Cl-.
In polar solvents such as MeCN, MeNO2 or CCl4, it is made of monomeric PCl5 molecules in association with a dimer.
No, PBr5 tends to change to a mixture of PBr3 and Br2 in the gas phase, whilst in the solid state, PBr5 is made of PBr4+ and Br- ions. The P-Br bond lengths in the PBr4+ ions are 213 to 217 pm.
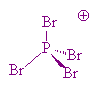 PBr4 |
Because they are bigger than chlorine atoms, it seems that six bromines cannot attach to the same phosphorus atom, there are too many non-bonding repulsions.
No one is sure if it exists. In 1978, it was reported to have been made, as black-brown crystals, from the reaction of PCl5 (in solution in CH3I) and MI (M = Li, Na or K). However, so far there are no confirmatory reports of its structure.
No, it's relatively simple. SbCl5, which is stable to 140°C, can readily be made from the reaction of SbCl3 and Cl2. However, whilst solid SbCl5 has the expected molecular structure at room temperature, below -54.1°C it changes reversibly to a dimeric molecule, Cl4Sb(μm-Cl)2SbCl4. In the SbCl5 molecules, Sb-Cl (axial) distances are 233.3 pm and Sb-Cl (equatorial) distances are 227.04 pm.
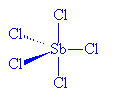 SbCl5 |
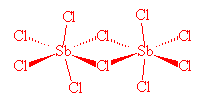 Sb2Cl10 |
Sb is a bigger atom than As, so it is not surprising that it finds it easier to accommodate six chlorine atoms round it. It is possible that AsCl5 could dimerise at very low temperatures, since six coordinate [AsCl6]- and [AsCl5(Me3PO)] species are known.
The effect has been ascribed to the stabilisation of a 4s2 electron pair in the elements following the 3d transition metals, caused by incomplete shielding of the nucleus lowering the energy of the 4s orbital and making it harder to promote 4s electrons. So AsCl3 is more stable than AsCl5. A similar reason has been given for other facets of the behaviour of these elements, such as the difficulty in making the perbromate ion.
The capacity of chemistry to surprise is not exhausted, whilst the skill and ingenuity of modern chemists in studying molecules under unfavourable conditions continues to know no bounds.
![]()
| PCl5 | J.J.Berzelius, Ann.Phys. (Leipzig), 1816, 53, 393. A.J.Balard, Ann.Chim.Phys. [2], 1826, 32, 374. |
| AsCl5 | K. Seppelt, Angew. Chem. Int. Ed. Engl, 1976, 15, 377. S. Haupt and K. Seppelt, Z. Anorg. Allgem. Chem., 2002, 628, 729. |
| SbCl5 | H. Rose, Pogg. Ann., 1825, 3, 443. A. Haagen, Pogg. Ann, 1867, 131, 122. |
| PI5 | N.G.Feshchenko, V.G.Kostina and A.V.Kirsanov, J. Gen. Chem. USSR, 1978, 48, 196.. |
| AsCl5 | S. Haupt and K. Seppelt, Z. Anorg. Allgem. Chem., 2002, 628, 729. |
| AsF5 | J. Köhler, A. Simon and R. Hoppe, Z. Anorg. Allgem. Chem, 1989, 575, 55 (solid). F.B. Clippard and L.S. Bartell, Inorg. Chem., 1970, 9, 805 (gas). |
| PF5 | D. Mootz and M. Wiebcke, Z. Anorg. Allgem. Chem., 1987, 545, 39. (solid). V.P. Spiridonov, A.A. Ischenko and L.S. Ivashkevich, J. Mol. Str., 1981, 72, 153 (gas). |
| PCl5 | B.W. McClelland, L. Hedberg and K. Hedberg, J. Mol. Str 1983, 99, 309 (gas) H. Preiss, Z. Anorg. Allgem. Chem., 1971, 380, 51 (solid). H.D.B. Jenkins, K.P. Thakur, A. Finch and P.N. Gates, Inorg. Chem., 1982, 21, 423 (metastable form). |
| PBr5 | H.M. Powell, J. Chem. Soc., 1942, 64. W. Gabes and K. Olie, Acta Crystallogr. Sect.B, 1970, 26, 443. |
![]()