![]()
Benzaldehyde
The smell of almonds and Maraschino cherries
![]()
![]()
Molecule of the Month June 2020
Also available: JSMol version.
![]()

An almond-flavoured Bakewell tart, decorated with Marascino cherries.
Photo: Bob Peters [CC BY 2.0]
BenzaldehydeThe smell of almonds and Maraschino cherries
Molecule of the Month June 2020
|
 An almond-flavoured Bakewell tart, decorated with Marascino cherries. Photo: Bob Peters [CC BY 2.0] |
Yes, you’re partly correct, but the type of almond matters here. Almonds are categorised as either sweet or bitter.
Bitter almonds (Prunus dulcis var. amara) are highly poisonous due to their high content of the molecule amygdalin (see MOTM November 2017), which breaks down when exposed to moisture to form the lethal hydrogen cyanide (see MOTM November 2011). Although the aroma of bitter almonds comes from several molecules (including benzaldehyde), the smell of HCN overpowers the other less pungent smells present and dominates the overall aroma - which readers of murder-mystery novels will recall as the tell-tale signs of a cyanide poisoning. Bitter almonds grow on trees, but tend to be a bit smaller and have pointier ends than their sweeter varieties. They usually give off a considerably strong scent and are often used to make non-edible products like soaps or perfumes. When cooked, bitter almonds lose their toxicity, but nevertheless the sale of the unrefined nuts is prohibited in the U.S. and many other countries.

The other type - sweet almonds (Prunus dulcis) – are not poisonous and are edible straight from the tree. They are sold in food shops and are often crumbled and sprinkled onto desserts or used in cakes. Sweet almonds have a much less intense aroma due to the absence of amygdalin, so now the aroma of benzaldehyde is what gives sweet almonds their characteristic smell. A closely related molecule, cinnamaldehyde, is responsible for the smell of cinnamon.
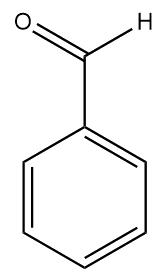 |
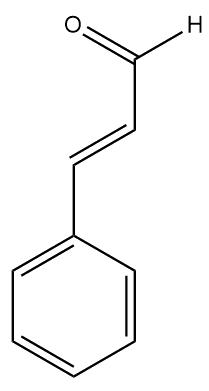 |
| Benzaldehyde | Cinnamaldehyde |
Ah, not just any cherries, but Maraschino cherries.
Maraschino cherries (named after the Croatian Marasca variety), often called 'cocktail cherries', are cherries which have been dyed red, impregnated with sugar, and packed in a sugar syrup that is flavoured with oil of bitter almonds, or a similar flavour. The key here, is that the oil has been processed (heated) to remove the HCN, leaving only the benzaldehyde – which is why these cherries smell of almonds.
 Photo: TheCulinaryGeek from Chicago, USA [CC BY 2.0] |
 Photo: Public domain via Wikimedia Commons |
 Photo: TheCulinaryGeek [CC BY 2.0] |
| A Maraschino cherry is often used to decorate ice-creams. Indeed, the expression "cherry on top" refers to the Maraschino cherries decorating the top of an ice cream sundae. They are also used on cakes, and, of course, in cocktails, like the Tequila Sunrise. |
||
 Is it used to flavour other things, too?
Is it used to flavour other things, too?Yes, as well as obvious almond foodstuffs like marzipan, Bakewell tart, and Amaretti biscuits, benzaldehyde is used to provide the characteristic cherry/almond smell of some lozenges, shampoos, and even soap. Industrially, it is used as a precursor to many organic compounds, especially analine dyes (such as malachite green) and acridine dyes, as well as many pharmaceuticals and plastics. It is even used as a bee repellent!
Bees do not like the smell of benzaldehyde, so beekeepers have exploited this to temporarily keep the bees away from the honeycombs while they extract the honey, with reduced risk of being stung. Benzaldehyde is painted onto a fumeboard (see photo, right) which is then put back onto the top of the hive causing the bees to retreat deeper into the hive. This allows the beekeeper to access the honey in the top honeycomb without fear of being stung. See a video of this. Benzaldehyde has also been reported to be a repellent for other insects, too, such as ants and fruit flies.
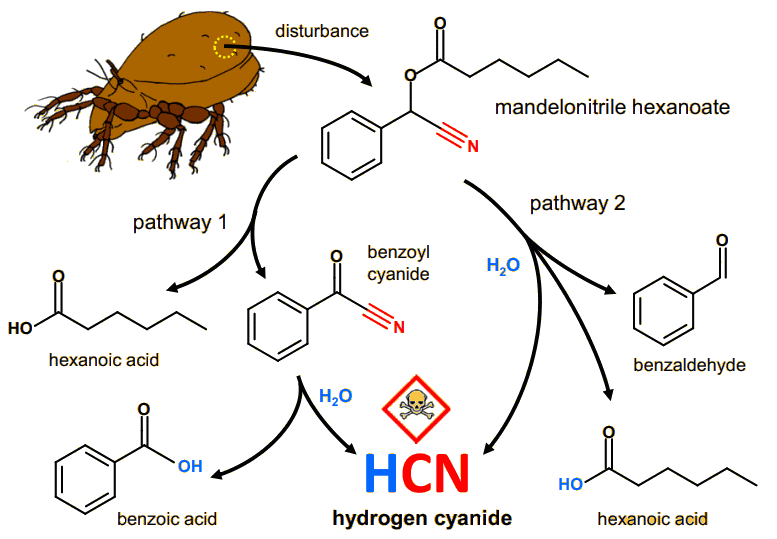
Apart from beekeepers, humans don't use benzaldehyde sprays to repel insects, preferring a more general-purpose repellent, such as DEET (see MOTM for January 2011). However, other insects do use it. Certain varieties of polydesmid millipedes exude benzadehyde as a means of deterring ant attacks. But other millipedes and mites go a stage further - they biochemically combine benzaldehyde, hexanoic acid and water to form a molecule called mandelonitrile hexanoate (MNH). This molecule is a safe way to store HCN. When threatened, another biochemical reaction reverses this process and the insect sprays HCN solution (prussic acid) at its attacker. According to one study published in 1936, it was common knowledge among natives of the island of Hispaniola (now called Haiti and the Dominican Republic), that large spirobolidan millipedes could blind chickens and other animals with their sprayed secretions. Indeed, a single Apheloria millipede weighing about 1 g can produce up to 600 μg of HCN, 18 times the lethal dose for a 300 g pigeon and 6 times that for a 25 g mouse!
 |
 |
| The biochemical pathway for HCN storage and release via MNH in the oribatid mite (Oribatula tibialis). Pathway 1: MNH is cleaved by a catalytic oxidation to benzoyl cyanide and hexanoic acid on the mite's body surface. Subsequently, benzoyl cyanide hydrolyses to benzoic acid and HCN. Pathway 2: Direct hydrolysis of the ester bond in MNH in the presence of moisture, resulting in the release of HCN, benzaldehyde, and hexanoic acid. [Figure taken from: A. Brückner,, G. Raspotnig, K. Wehnera, R. Meusinger, R.A. Nortond, M. Heethoff, Storage and release of hydrogen cyanide in a chelicerate (Oribatula tibialis), PNAS 114 (2017) 3469.] |
Apheloria virginiensis corrugata miilipede. [Photo: Marshal Hedin, San Diego State University [CC BY 2.0 - Wikimedia Commons] |
Well, humans breath out tiny amounts of benzaldehyde as a result of various metabolic and biochemical processes occurring the body, one example being lipid peroxidation. When these processes go wrong, as can happen in certain forms of cancer, the level of exhaled benzaldehyde increases. Sensors for the selective detection of benzaldehyde and other aldehydes from breath samples are currently being developed, and may allow early detection of disease. However, such breath sensors may need to measure simultaneously a combination of many different exhaled biomarker molecules, not just benzaldehyde, in order for them to make a reliable and accurate diagnosis.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.9746543]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.9746543]