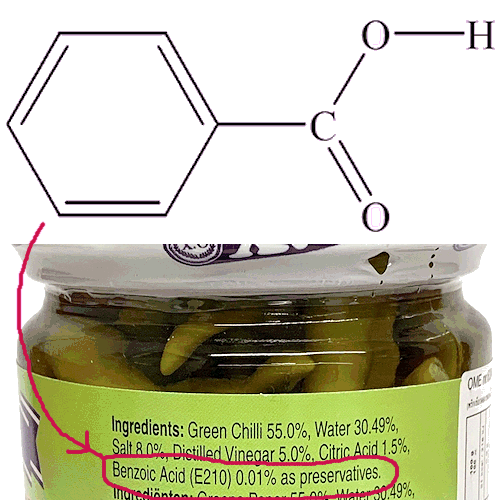
![]()
Benzoic Acid
Not just an E number!
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month May 2023
Also available: JSMol version.
![]()
 |
Benzoic AcidNot just an E number!
Simon Cotton
Molecule of the Month May 2023
|
 E number, what does that mean?
E number, what does that mean?E numbers are a code used by the European Union to identify food additives. Thus, benzoic acid is referred to as E210 when it is used as a food additive.
It helps prevent the growth of microorganisms like yeast, and works against moulds and bacteria.
It is believed to be active against cell membranes. That said, usually it is the salts of benzoic acid that are used, because they are more soluble - most often the sodium salt, more rarely the potassium salt (for people on low-sodium diets). Sodium benzoate is E211 and potassium benzoate E212 (calcium benzoate is E213).
It seems not. The closely related salicylic acid (2-hydroxybenzoic acid) is synthesised in many plants and has an important role in plants’ defence against pathogens. It is of course the active molecule in aspirin (MOTM Feb 1996). Acetic acid, notably in vinegar, is used as a preservative, as it retards bacterial growth.
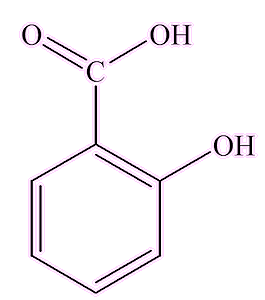 |
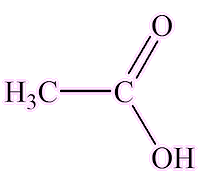 |
| Salicylic acid | Acetic (ethanoic) acid |
 Who discovered it?
Who discovered it?It was first mentioned by Nostrodamus (image, right) in 1556, who although being more famous for his prophesies, was also a physician and apothecary. He described heating up gum benzoin and then collecting and condensing the gases into a solid product. When organic chemistry developed in the 19th century, other scientists, notably Justus von Liebig (who chemists will recognise from the Liebig condenser), determined its composition.
 What's gum benzoin?
What's gum benzoin?It's the resin (solidified sap) from the bark of several species of tropical Styrax trees (such as Styrax benzoin and Styrax benzoides). Because of its sweet vanilla-like aroma it is used in perfumes and incense, especially in Orthodox Christian churches in Russia. It's also used as a flavouring in alcoholic drinks, and in medicine (dissolved in ethanol) to protect the skin under a bandage or cast and diminish itching.
[Chemists may have also heard of an organic compound called benzoin; confusingly this is unrelated to gum benzoin, although it can be made from it.]
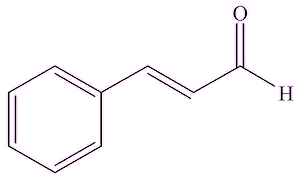
It's still extracted from gum benzoin, which these days is mostly obtained from Sumatra and Laos. Apart from benzoic acid, other well-known organics found in these gum benzoin resins include vanillin (MOTM February 2008), cinamaldehyde (MOTM August 2006) and cinnamic acid.
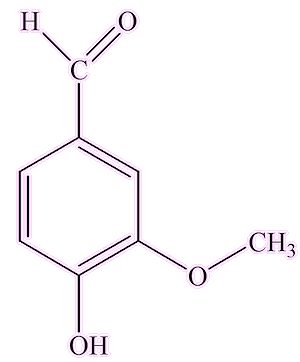 |
 |
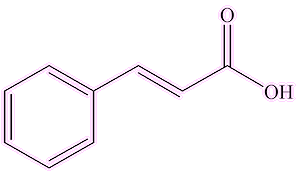 |
| Vanillin | Cinnamaldehyde | Cinnamic acid |
But benzoic acid and its salts (benzoates) also crop up naturally in a number of fruits, such as strrawberries, and has a high concentration in many berries such as cranberries, bilberries and blueberries. It is also found in some herbs and spices, such as cloves, thyme, nutmeg, star anise and cinnamon. It can also be found in milk, yoghurt and some cheeses.

Foods high in benzoic acid: blueberries (up to 1300 mg kg-1), cinnamon (335 mg kg-1), cottage cheese (90 mg kg-1).
[Images: Wikimedia Commons]
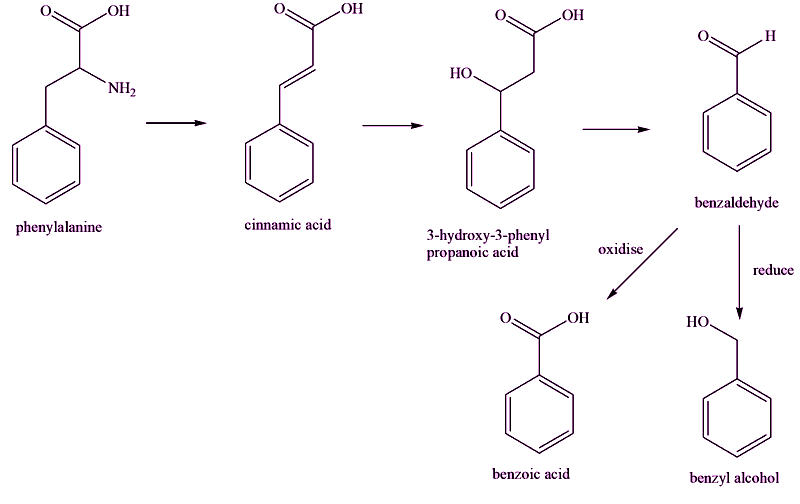
More than one pathway is involved, each involving a number of reactions catalysed by enzymes, but some key compounds are shown here. The starting point is the amino-acid phenylalanine, which undergoes changes to its sidechain, forming successively cinnamic acid and 3-hydroxy-3-phenylpropanoic acid. In the next step, the sidechain is shortened, forming benzaldehyde. Different enzymes then convert benzaldehyde into benzoic acid, by oxidation, or benzyl alcohol, by reduction.
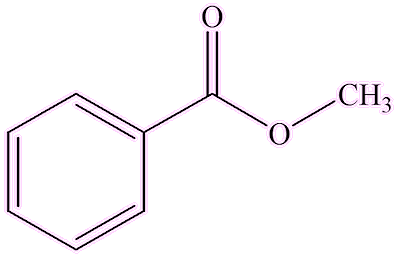
Other enzymes change benzoic acid into different compounds, like esters. A methyl transferase enzyme replaces the hydrogen atom of the carboxylic acid group by a methyl group, forming the ester methyl benzoate. A different enzyme catalyses the reaction between benzoic acid and benzyl alcohol, forming another ester, benzyl benzoate. Both these esters, especially benzyl benzoate, are found, along with benzoic acid, in gum resin.
 |
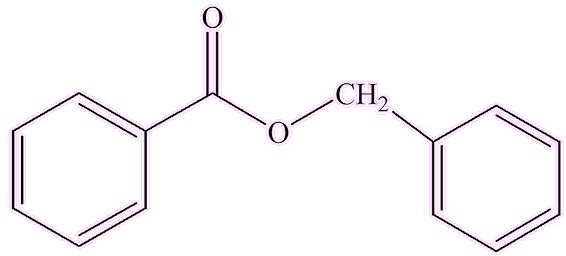 |
| Methylbenzoate | Benzylbenzoate |
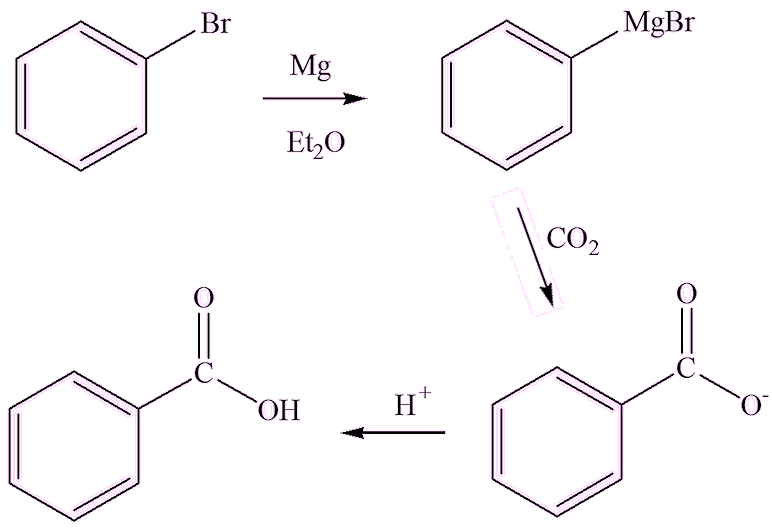
A ‘classic’ method starts with bromobenzene. First it is converted into a Grignard reagent by reaction with magnesium turnings in dry ether; this is then poured onto dry ice, the CO2 being the source of the carboxylate group. On acidification, benzoic acid is formed. Voila! But it is much cheaper to buy the commercial product!
Other routes include the oxidation of phenylacetylene with ozone and trifluoroacetic acid.
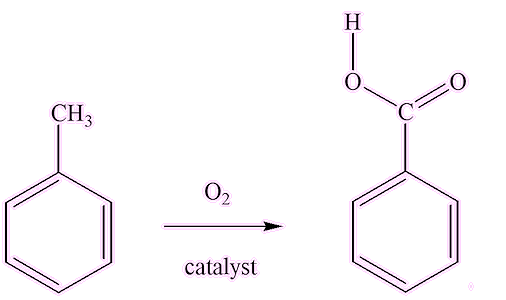
Industry uses methods like the catalytic oxidation of toluene, using catalysts containing a variety of transition metal ions (e.g. Mn(IV), Co(II), V(IV)).
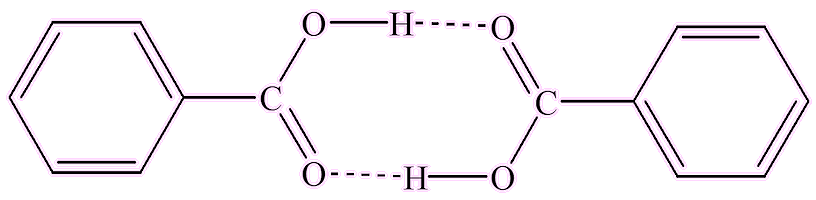
Well, it is a substance that crystallises nicely from hot water, as it is not very soluble in cold water. Its melting point of 122°C is a ‘standard’ that can be used to calibrate melting point apparatus. The structure in the crystals features pairs of benzoic acid molecules hydrogen-bonded together.
Yes, in the gas phase, ethanoic (acetic) acid is also composed of dimers. However, in the solid state, ethanoic acid has a chain structure.
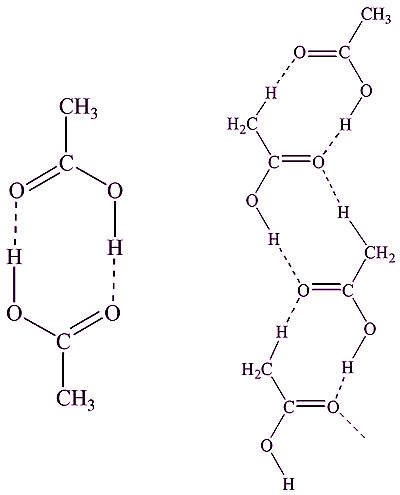
Structures of (left) acetic acid dimers (gas phase) and (right) chains (solid phase).
Back in 1993, it was discovered that benzoic acid can be converted into benzene, a known carcinogen, when it reacts with ascorbic acid (Vitamin C - MOTM July 2017) in the presence of certain transition metal ions (e.g. Cu2+, Fe3+). Vitamin C is an antioxidant, and can also be found in food. Ever since then people have kept a careful watch, but benzene levels in beverages have been found to be well below 5 ppb, if it can be detected at all.
Is benzoic acid used for anything besides food preservatives?It's preservative action doesn't end with foodstuff - benzoic acid is used to prevent bacteria and fungal build-up in preservative in many pharmaceutical drugs, baby products, skin products, cleansing products, hair and nail products, soaps, bath products, detergents, toothpaste, jam, beverages, poultry, agriculture, perfume, dyes,...the list goes on. It has a a medical use. It's been known for nearly 150 years that benzoic acid has antifungal properties, and this knowledge probably helped British dermatologist Arthur Whitfield to develop an ointment to treat fungal skin infections such as ringworm and athlete's foot. Whitfield's Ointment contains 6% benzoic acid with 3% salicylic acid in a base of lanolin or petroleum jelly, and although developed nearly 120 years ago is still in use today.
|
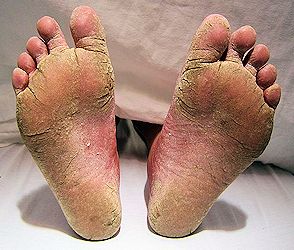 A severe case of athlete's foot. [Image: James Heilman, MD, CC BY-SA 3.0 via Wikimedia Commons] |
But one of its most important uses is as a precursor to phenol, via the reaction:
C6H5COOH + ½O2  C6H5OH + CO2
C6H5OH + CO2
Phenol is a hugely important industrial chemical, being itself the precursor to many other products including plastics, polycarbonates, epoxies, detergents, herbicides, numerous pharmaceutical drugs and Nylon (MOTM June 2010).
Methyl benzoate isn’t just any ordinary ester; it is quite versatile. You can make it in the lab by the usual acid-catalysed esterification of benzoic acid with methanol (the classic "Fischer synthesis"). It has a fruity-spicy smell.
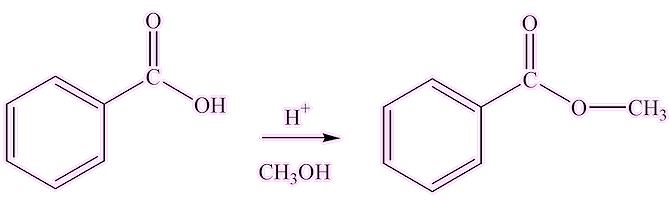
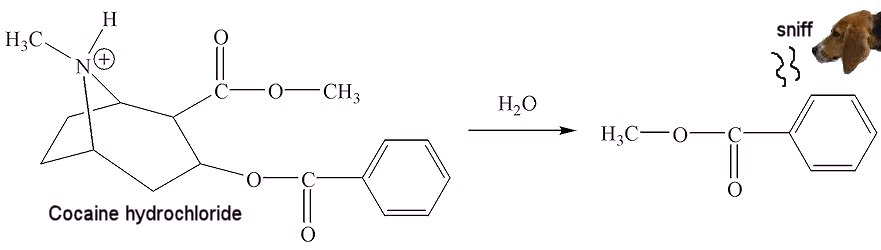
It is emitted by some flowers, such as snapdragon and petunia, to attract insects like bees for pollination. It is toxic to certain other insects, and it has been suggested that it may act as a pesticide. Methyl benzoate is also formed when cocaine hydrochloride is hydrolysed by traces of moisture in air - police 'sniffer dogs' are trained to detect the smell of methyl benzoate.
Benzyl benzoate isn’t as versatile, but on account of its involatility (b.p. ~320°C) it makes a good perfume fixative.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.21300885]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.21300885]