The Crystal Structures of Caeruloplasmin and Related Metalloproteins
The structure of the protein shows six domains, a feature which
was discovered over twenty years ago by an electron microscope investigation. This study also established the
molecular weight of 130kD.
 The six domains are arranged in a triangular array, and each domain has a
beta-barrel with strands like those in other cupredoxins such as
azurin or plastocyanine. In the diagram
the even barrels are coloured blue, the odd ones yellow. {Diagram after
Zaitseva et al}
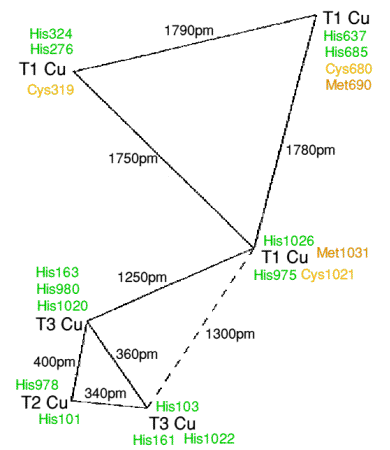
The X-ray crystal structure shows that
there are also six non-labile copper atoms. Three of these form a
trinuclear cluster (like that in ascorbate
oxidase) and situated at the interface of domains 1 and 6. The coppers are only
coordinated to histidine nitrogens. The trimer is made from a T3 copper pair
and a T2 copper, and
as in ascorbate oxidase a hydroxide bridges the T3 pair, and a single hydroxide
sits on the T2 site. The human caeruloplasmin structure has a very strong kinship
with the structure of ascorbate oxidase, with a T1 copper at 1200-1300pm.
Nitrite reductans, another
copper oxidase, has a similar three domain structure. {Multi-copper is certainly
appropriate with nitrite reductans} The three other T1 coppers are in the even
domains 2,4 and 6. Two of the T1 coppers have four coordination, with two
nitrogen abd two sulphur ligands. The other T1 copper is trigonally coordinated,
more like plastocyanin, however there is a leucine residue which
replaces the methionines of the other two T1 coppers, but at a van der Waals
contact distance.
The six domains are arranged in a triangular array, and each domain has a
beta-barrel with strands like those in other cupredoxins such as
azurin or plastocyanine. In the diagram
the even barrels are coloured blue, the odd ones yellow. {Diagram after
Zaitseva et al}
The X-ray crystal structure shows that
there are also six non-labile copper atoms. Three of these form a
trinuclear cluster (like that in ascorbate
oxidase) and situated at the interface of domains 1 and 6. The coppers are only
coordinated to histidine nitrogens. The trimer is made from a T3 copper pair
and a T2 copper, and
as in ascorbate oxidase a hydroxide bridges the T3 pair, and a single hydroxide
sits on the T2 site. The human caeruloplasmin structure has a very strong kinship
with the structure of ascorbate oxidase, with a T1 copper at 1200-1300pm.
Nitrite reductans, another
copper oxidase, has a similar three domain structure. {Multi-copper is certainly
appropriate with nitrite reductans} The three other T1 coppers are in the even
domains 2,4 and 6. Two of the T1 coppers have four coordination, with two
nitrogen abd two sulphur ligands. The other T1 copper is trigonally coordinated,
more like plastocyanin, however there is a leucine residue which
replaces the methionines of the other two T1 coppers, but at a van der Waals
contact distance.
The six copper atoms are arranged into two large triangles, however the
triangles are not in the same plane, a feature best seen
via the crystal structure. In addition to the six coppers there are also two
additional labile coppers. Some progress has been made in establishing the
structure-function relationships
for the caeruloplasmins.