![]()
Chloromethane
A natural ozone destroyer
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month July 2024
Also available: JSMol version.
![]()

|
ChloromethaneA natural ozone destroyer
Simon Cotton
Molecule of the Month July 2024
|  |
Wrong in more than one way.
CFCs, such as CF2Cl2 (MOTM June 2005 and CFCl3 MOTM June 2019) all contain fluorine, whereas chloromethane doesn't. Secondly, it is much more reactive than CFCs, so doesn’t hang about in the atmosphere for so long.
No, it is not an insecticide either.
Firstly, chloromethane is simply a methane molecule where one of the hydrogens has been replaced by a chlorine. Most chloromethane is released into the atmosphere from natural sources.
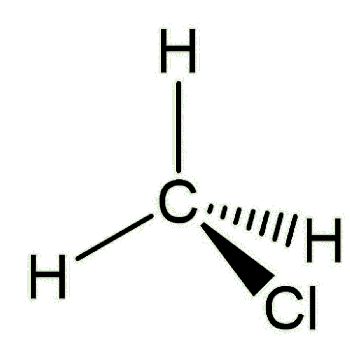 |
| Chloromethane a.k.a. methyl chloride |
Not so, but it is only recently that their number has been appreciated. Less than a dozen natural organic compounds containing chlorine were known in 1954 – such as chlorotetracycline (see MOTM September 2011), but this number has now risen to over 2500, as scientists have found better ways of detecting them.
The most abundant of these compounds is CH3Cl, chloromethane, a gas at normal temperatures (its b.p. is -24°C). The great eco-guru James Lovelock detected CH3Cl in the air over southern England in 1974-1975. It has been estimated that 5 × 106 tonnes of CH3Cl are released globally into the atmosphere each year, with over 90%, maybe even 99%, being from natural sources.
Well, until around the mid-1990s, it was assumed that CH3Cl came primarily from warm oceans. But now it is known that the oceans contribute at most 11% of the world’s chloromethane, and that there are many terrestrial sources.
Biomass burning, for a start, forest and brush fires. It is reckoned that there are 200,000 lightning-triggered fires across the world each year. Of course, there are fires created by human activity, but they contribute much less. Wood-rotting fungi also produce chloromethane. Then there are salt marshes, mangroves and peat bogs worldwide, major emitters of CH3Cl. For example, a halophytic plant that grows abundantly in salt marshes called Batis maritima (pickleweed) produces chloromethane. And it is calculated that tropical plants may emit around half the chloromethane produced worldwide.
 |
 |
| Biomass burning [Image: Ramos Keith, U.S. Fish and Wildlife Service, Public domain, via Wikimedia Commons] |
Wood-rotting fungi [Image: André Lage Freitas, CC BY-SA 3.0 via Wikimedia Commons] |
 |
 |
| Pickleweed (Batis maritima) [Image: Forest & Kim Starr, CC BY 3.0 US via Wikimedia Commons] |
A peat bog [Image: Peat Bog on Yockenthwaite Moor by John H Darch, CC BY-SA 2.0 via Wikimedia Commons] |
People are still working these out. One important source of CH3Cl at the molecular level is believed to be pectin. This is a biopolymer, a polysaccharide, that is widely found in plant cell walls, and is thought to supply methyl groups, using a SN2 reaction between chloride ions and a methoxy group in pectin – and also lignin.
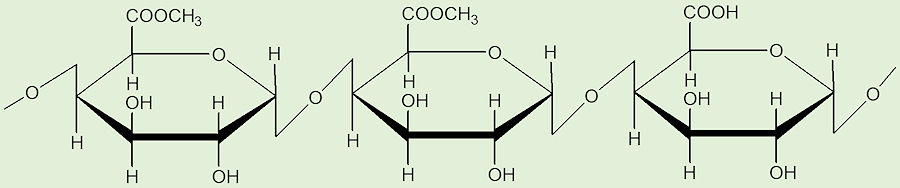
Pectin
Are there other sources of chloromethane?One perhaps unexpected source of CH3Cl is volcanic eruptions; it was first noted among emissions in the 1969 eruption at Santiaguito in Guatemala. Since then it has been detected in many others, such as the 1980 eruption of Mount St. Helens, in the state of Washington, USA, which also released CH3Br and CH3I; and more recently at Etna, in Italy. But their contribution is negligible overall. |
 Mount St Helens in 1982 [Image: Lyn Topinka, Public domain via Wikimedia Commons] |
 comet 67P/Churyumov–Gerasimenko (67P/C-G) in 2021 [Image: NASA/JPL-Caltech/ZTF, Public domain, via Wikimedia Commons] |
And?Then there is chloromethane in outer space. Really?Chloromethane was the first organochlorine compound to be detected in outer space – as both isotopologues (CH335Cl and CH337Cl) - in the coma of comet 67P/Churyumov–Gerasimenko (67P/C-G), and also, some four hundred light years away, within several young star clusters. |
The atmosphere contains CH3Cl at a concentration of some 0.6 ppb, so it contributes far more chlorine than CFCs like CF2Cl2. On the other hand, the greater reactivity of CH3Cl ensures a shorter atmospheric residence time (1.4 years compared with 100 years for CF2Cl2). About 20 years ago it was estimated that chloromethane contributed around 15% of ozone-depleting emissions; this proportion is bound to increase as anthropogenic emissions of CFCs decrease.
Chloromethane acts as an ozone depleter in a similar way to CFCs, by acting as a source of chlorine radicals, which catalyse the decomposition of ozone.
CH3Cl  •CH3 + •Cl
•CH3 + •Cl
•Cl + O3  •ClO + O2
•ClO + O2
•ClO + O 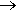 •Cl + O2
•Cl + O2
The process regenerates chlorine atoms (radicals), so one chlorine atom can destroy thousands of ozone molecules.
Chloromethane is not believed to be a significant ‘greenhouse gas’.
One traditional way, first reported by M. Berthelot in 1858, is by the reaction of methane with chlorine, in the presence of ultraviolet light, which generates reactive chlorine atoms by homolytic fission of the Cl-Cl bond. The resulting chlorine atom abstracts a hydrogen atom from a methane molecule, generating a methyl radical (•CH3), which in its, turn abstracts a chlorine atom from a chlorine molecule. This forms a molecule of chloromethane and in its turn regenerates a chlorine radical, so the cycle can continue.
Overall: CH4 + Cl2 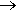 CH3Cl + HCl
CH3Cl + HCl
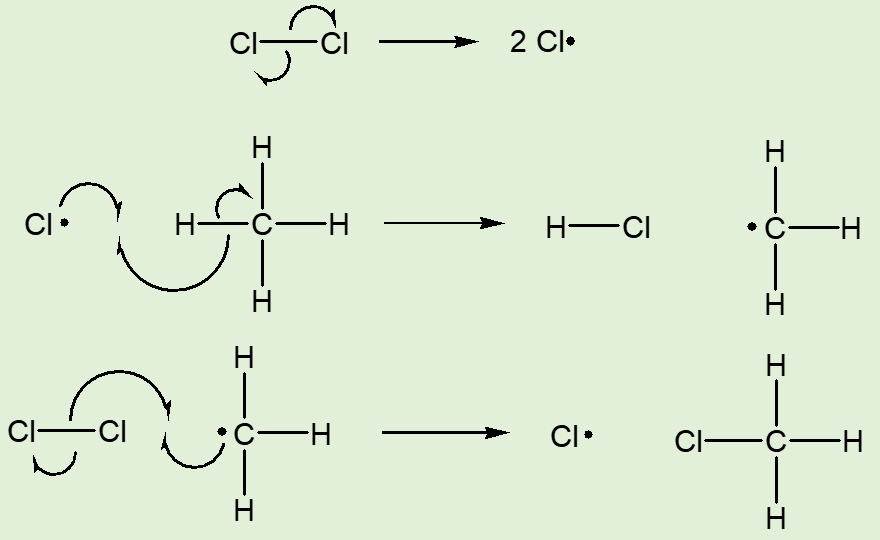
Synthesis of CH3Cl
Apart from the use of elemental chlorine, a very real disadvantage of this method is that further substitution can follow, with the additional formation of CH2Cl2, CHCl3 and CCl4, causing the need for purification of the product.
But there are better ways, e.g. from methanol by reaction with HCl, possibly generated in situ from the reaction of NaCl with conc. sulfuric acid. This is how chloromethane was originally made in 1835, by pioneering French organic chemists Dumas and Peligot.
 |
 |
| Jean Baptiste André Dumas [Image: Public domain via Wikimedia Commons] |
Eugène Peligot [Image: Gaspard Félix Tournachon (1820-1910), Public domain, via Wikimedia Commons] |
The mechanism involves the protonation of the –OH group, which creates an incipient water group, a much better ‘leaving group’ than hydroxide, OH-. The intermediate cationic species can then undergo nucleophilic attack by a chloride ion, displacing the water molecule and forming chloromethane.
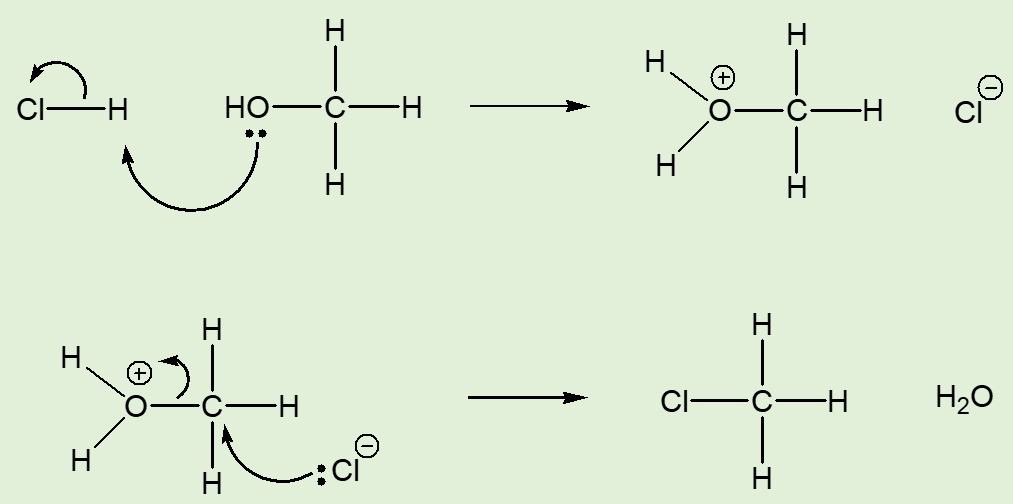
Alternative synthesis of CH3Cl
It used to be used in refrigerators, as it had similar properties to Freons, but no longer. Another former use was in making the anti-knock agent Pb(CH3)4 (similar to Pb(C2H5)4 MOTM Jan 2001), as a fuel additive – again, no more. It’s used to synthesise methylchlorosilanes, such as (CH3)2SiCl2, important in making silicone polymers.
 |
 |
 |
| Silicone soup ladles [Image: GOttawaAC, CC BY-SA 3.0 via Wikimedia Commons] |
Flexible ice cube trays made of silicone [Image: Gmhofmann, CC BY-SA 3.0 via Wikimedia Commons] |
Silicone brush used for basting liquids when cooking [Image: Evan-Amos, Public domain via Wikimedia Commons] |
It is also used in methylating –OH groups, for example in making methylcellulose; and in organic synthesis for a range of substances like methanethiol CH3SH (MOTM May 2017) and methylamines - CH3NH2, (CH3)2NH and (CH3)3N (MOTM August 2004). So it is useful in industrial synthesis, but the industrial production, around a million tons a year, is well below the natural level.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.24340675]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.24340675]