Cucurbituril
by
Fabio Pichierri


Molecule of the Month - March 2006
Also available: Chime Enhanced, JMol, and VRML versions of this page.
Cucurbituril
(from cucurbita = pumpkin) is the fancy name given to
the pumpkin-shaped macrocycle hexamer
obtained from the condensation reaction between glycoluril
and formaldehyde (in excess):

The story of cucurbituril, hereafter
referred to as CB or CB[6], starts in 1905 (Einstein's
magic year!!!) when three German chemists, Behrend,
Meyer, and Rusche, published a paper on the
illustrious Liebigs Annalen der Chemie 339 (1905) 1.
Their paper described the reaction between glycoluril
and an excess of formaldehyde (see the above Scheme) which yielded a
cross-linked polymer (Behrend's polymer) that upon
treatment with concentrated sulfuric acid produced a crystalline precipitate. Behrend characterized the crystalline precipitate as C10H11N7O4x2H2O.
At that time, however, chemists could not make use of analytical techniques such
as x-ray crystallography that are nowadays routinely employed to identify the atomic
structure of molecules (note that in 1912, seven years later the publication of
Behrend's paper, Max von Laue
observed the first diffraction pattern from crystals).
The molecular structure of CB remained unknown for about 76 years up
until 1981 when a team of three American chemists working at the University of
Illinois, Chicago, decided to repeat the 1905 synthesis of Behrend
et al. Treatment of the precipitate (Behrend's
polymer) with H2SO4 (conc.) and subsequent dissolution in
hot water afforded a crystalline solid which, after being subjected to a
single-crystal x-ray structure analysis, revealed the beautiful pumpkin-shaped
molecule shown in Figure 1. In reference (4) of their landmark paper the
authors state: "The trivial
name cucurbituril is proposed because of a general
resemblance of CB to a gourd or pumpkin (family Cucurbitacee),
and by devolution from the similarly named (and shaped) component of the early
chemists' alembic". And another nice
chapter of the history of Chemistry was written!
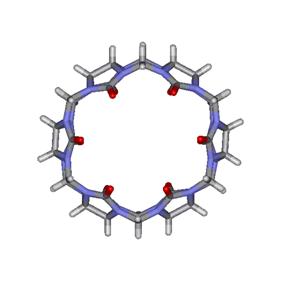
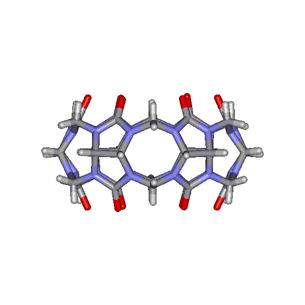
Figure
1. Molecular structure of cucurbituril:
top (left) and lateral (right) views. (Click image for 3D structure).
The molecular dimensions of CB are as follows: the internal and external
diameters are at 3.9 Å and 5.8 Å, respectively, the height at 9.1 Å and the
volume corresponds to 164 Å3. In 1984, only three years after the
publication of the JACS paper, Freeman publishes the crystal structure of the
first host-guest complex of CB[6] which incorporates
the p-Xylylenediammonium
cation into the macrocycle's
cavity. This structure, along with those of 11 other adducts also reported in
this study, firmly established the cavitand-like
character of CB. Note from Figure 2 that each N-H moiety forms a pair of H-bond
interactions with two carbonyl (C=O) oxygen atoms on opposite portals.
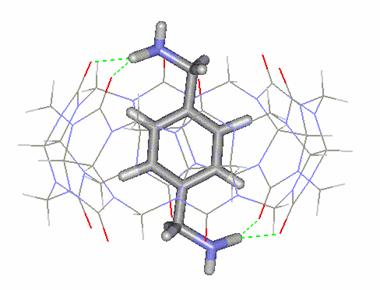
Figure
2. Molecular structure of the CB[6]:p-Xylenediammonium
complex
After the exciting discoveries of the early 80s, not much happened
in the field of cucurbituril chemistry for a certain number
of years. In the year 2000, however, another important breakthrough: Kimoon Kim and his group at Pohang
University of Science and Technology (South Korea) present the syntheses and
crystal structures of three CB homologues, namely
CB[5], CB[7], and CB[8]. The crystal structure of CB[10]
has been subsequently characterized by Day's group in 2002 (as its CB[5]@CB[10]
complex – vide infra) and in 2005 by Isaacs's group (cucurbituril-free
CB[10]) whereas those of CB[9] and CB[11] are yet to be determined. Note,
however, that evidence about their existence is supported by the results of electrospray ionization mass spectrometry (EIMS)
experiments.
Given the unique shape of the CB[n] molecules, it is perhaps not
surprising that a large variety of interesting supramolecular
architectures have been so far discussed in the scientific literature. Figure 3
shows an example of molecular necklace that results
from the threading of three CB[6] macrocycles
onto a molecular ring containing three Pt metal centres.
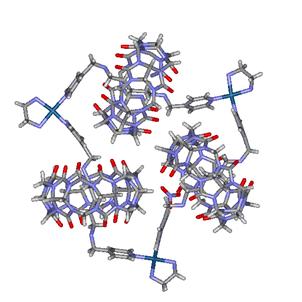
Figure 3. Molecular necklace made of three CB[6] units as beads
The space provided by the CB's cavity can be exploited to host
selected molecular pairs, as shown in Figure 4. The aromatic molecules brought
in close proximity can thus be activated either electrochemically or photochemically to yield products that are likely to differ
from those obtained without the presence of the macrocycle.
Hence, cucurbituril macrocycles
can be employed as molecular nanochambers or nanoreactors.
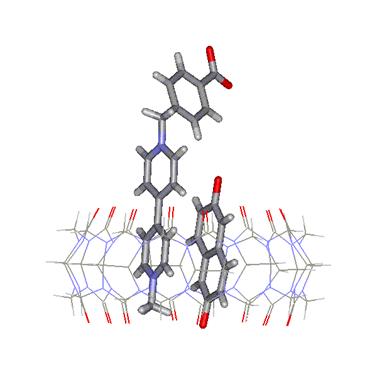
Figure
4. A molecular pair inside the cavity of CB[8]:
an example of nanoreactor
Another interesting application of CBs is in the construction of molecular
machines. According to Balzani and coworkers, "a molecular-level machine can be identified as
an assembly of a distinct number of molecular components that are designed to perform
machine-like movements (output) as a result of an appropriate external stimulation
(input)." In this regard, Day and coworkers have succeeded in characterizing
the structure and solution properties of the CB[5]@CB[10] complex shown in
Figure 5. Because in solution CB[5] freely rotates inside
the cavity of CB[10], the authors classified this complex as a molecular
gyroscope (or gyroscane) in analogy with
macroscopic devices that are employed to measure and maintain orientation
during flight.
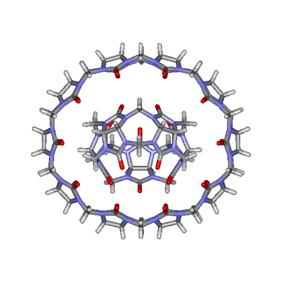
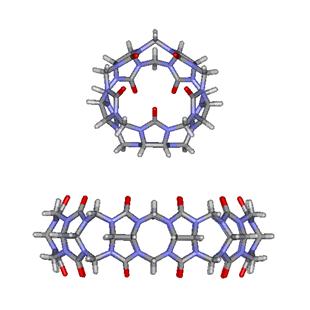
Figure
5. Molecular gyroscope made of the CB[5]@CB[10] complex
One of the main problems encountered with CB is its poor
solubility in water. In this regard, Kim and coworkers have introduced a pair
of hydroxyl groups (-OH) on each glycoluril unit as
shown in the modified CB[6] macrocycle
on the left side of Figure 6. This perhydroxylated CB[6] derivative shows good solubility in DMSO and can be further
modified with groups that can increase its solubility in water. The same group was
also able to introduce a carbon chain made of four methylene
moieties attached to each glycoluril unit, as shown on
the right side of Figure 6.
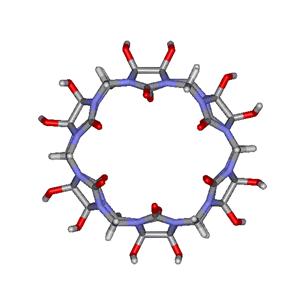
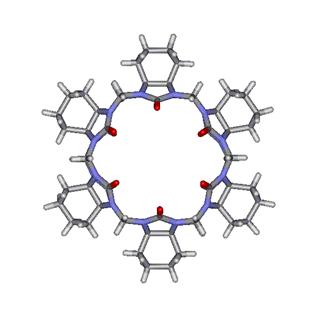
Figure
6. Modified CB[6] macrocycles
An interesting modification of CB is the replacement of its
carbonyl oxygen atoms (C=O) with sulfur atoms (C=S) which yields the thia-CB[6] analogue. Computer-aided design studies have
shown that thia-CB
macrocycles can be soldered with transition metal
ions such as Pd(II), Pt(II), or Hg(II) to produce
molecular nanotubes of any desired length and selected
diameters as the one shown in Figure 7. These new nanostructures, dubbed cucurtubes, might be useful in the development of
future molecular-scale wires for nanoelectronics.
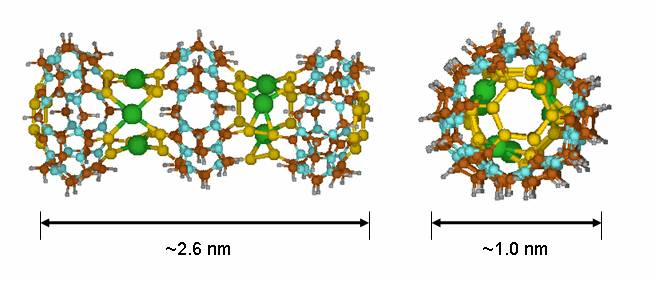
Figure
7. Structure of a cucurtube trimer (green spheres represent Hg2+ ions)
References
R. Behrend, E. Meyer, F. Rusche,
Liebigs Annalen der Chemie 339 (1905) 1 is the original report about CB
W.A.
Freeman, W.L. Mock,
N.-Y. Shih, J. Am. Chem. Soc.
103 (1981) 7367 reports the first ever crystallographic
characterization of the molecular structure of cucurbituril,
CB[6]
W.A. Freeman, Acta Cryst. B 40 (1984) 382 describes the
host-guest complexes of CB[6]
J. Lagona, P. Muchophadyay,
S. Chakrabarti, L. Isaacs, Angew. Chem. Int. Ed. 44 (2005) 4844 is the most
comprehensive review written on the cucurbit[n]uril
family of macrocycles – a must for the CB fans!
J. Kim et al, J. Am. Chem.
Soc. 122 (2000) 540 describes then syntheses and crystal
structures of CB[n], with n=5,7,8
A.I. Day et al., Angew. Chem. Int. Ed. 41 (2002) 275 describes the molecular-scale gyroscope made of the CB[5]@CB[10]
complex
F. Pichierri, Chem. Phys. Lett. 390 (2004) 214
reports the results of the first DFT calculations on CB[6]
and its sulfur analogue, thia-CB[6]
F. Pichierri, Chem. Phys. Lett. 403 (2005) 252 presents the computer-aided design of
tubular nanostructures obtained from the nanoscale
soldering of thia-CB[6] units with transition metals
V.
Balzani et al., Angew. Chem. Int. Ed. 39 (2000) 3348 an excellent
review on molecular-scale machines
The crystal structures depicted on this page were retrieved from
the Cambridge Structural Database (CSD) maintained by the Cambridge Crystallographic
Data Centre (CCDC), 12 Union Road, Cambridge, CB2 1EZ, UK
◊◊◊◊◊
I dedicate
this MOTM page to my lovely family.