![]()
Eribulin (Halaven)
The anti-cancer drug made from a sea-sponge
![]()
Paul May
University of Bristol
![]()
Molecule of the Month April 2011
Also available: HTML version.
![]()

|
Eribulin (Halaven)The anti-cancer drug made from a sea-sponge
Paul May
Molecule of the Month April 2011
|
 |
Yes. In 1986, two Japanese chemists Hirata and Uemura isolated a naturally-occurring compound from the marine sponge Halichondria okadai (picture above, right). The compound was named Halichondrin B, and it immediately began to generate great excitement when it was realised that it was extremely potent at killing certain types of cancer cells in small-scale tests. As a result of this discovery, it was immediately given top priority to be tested against a wide range of other cancers, and became one of the first compounds to be evaluated using the novel 60-cell line method developed by the US National Cancer Institute (NCI). In this technique, 60 different types of human tumor cells (including leukemia, melanoma and cancers of the lung, colon, brain, ovary, breast, prostate, and kidney) are tested with the potential anti-cancer molecule delivered at a single dose of 10 μM concentration. This process can be run in parallel, with dozens of different molecules being tested against all 60 cancer cell lines at the same time in a huge array. Any molecules which exhibit significant growth inhibition are prioritised, and the test repeated on them, but this time at five different concentration levels.
|
Unfortunately, the concentration of Halichondrin B in the sea sponge wasnít enough to enable commercial production for use in chemotherapy. For example, a ton of sea sponges could only produce 300 mg of Halichondrin B! The race was on to try to synthesise Halichondrin B in the lab, which wasn't easy due to its large size (molecular weight 1110) and complex structure. However, only 6 years later, T.D. Aicher and coworkers, chemists at Harvard University, published the complete chemical synthesis of this molecule.
In a way. Although the synthesis was a great achievement, Halichondrin B was still far too complex and the sythesis route too expensive to do on a large scale. The molecule needed to be stripped down to its essential components, while keeping, or even improving, its anti-cancer efficacy. Many tests were performed, but eventually the work led to te development of the structurally-simplified and pharmaceutically-optimized analog, which was named Eribulin [3,4]. Eribulin mesylate was approved by the U.S. Food and Drug Administration in 2010, to treat patients with metastatic breast cancer [5], and it is currently being marketed by Eisai Co. under the trade name Halaven [5]. It is also being investigated for use in a variety of other solid tumors, including lung cancer, prostate cancer and sarcoma [6].
|
Eribulin is classed as a mitotic inhibitor, which means it blocks the process of cell division and reproduction (mitosis). If the cells that are targeted are cancer cells, and these are prevented from dividing for prolonged periods, they 'self-destruct' in a process called apoptosis (programmed cell-death).
 |
 |
 |
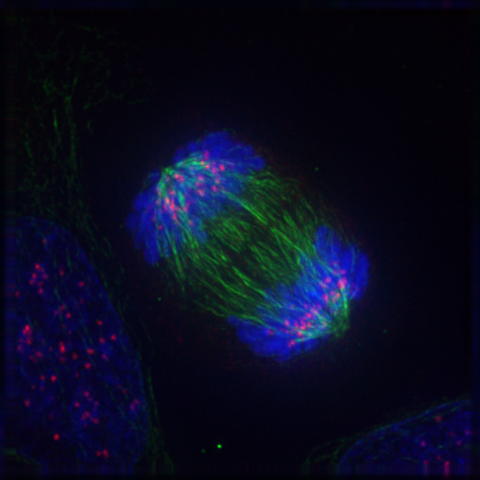
Mitosis in a cell. Microtubules (green) push the two sets of chromosomes (blue) further apart. |
|
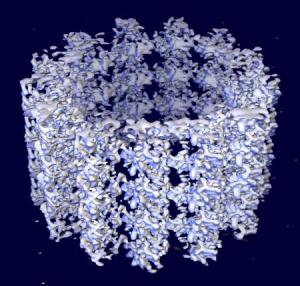
Space-filling model of a microtubule segment. The microtubule (+)-end is towards the top of the image. |
Within cells there are structures called microtubules (above, right), which are polymers made from a protein called α- and β-tubulin (above, centre). Microtubules are part of the cytoskeleton (the structural network) within the cell's cytoplasm. But as well as structural support, microtubules take part in many other processes. They are capable of growing and shrinking in order to generate force, and an example of this is the mitotic spindle that separates the chromosomes into two sets of pairs at either end of the cell during mitosis (above, left). During the early stages of mitosis, many microtubules increase in length by attachment of more tubulin dimers to one end, and grow out from the spindle for long distances (10 μm) into the cell searching for an unattached chromosome. If none is found, the microtubule loses dimers and shrinks again. This expansion and retraction is repeated many times, until eventually it meets and chemically attaches to a chromosome. When every chromosome has been 'fished' by a microtubule in this way, they are collected into the correct order and then separated into 2 halves to divide the cell in two (see image above, left). However, if even one of the many chromosomes in the cell fails to attach to a microtubule, the cell then realises something hss gone wrong, and undergoes cell death rather than divide incorrectly.
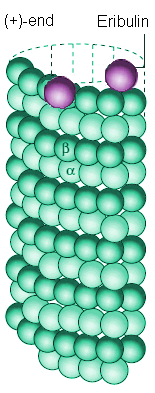 |
Eribulin binds to sites at the (+)-end of a microtubule |
Many anti-cancer drugs (such as Paclitaxel (Taxol), Combretastatins, Vinblastine (Velban), etc.) work by chemically bonding to sites along the microtubule structure, either in the centre, or at one end, disrupting the growth and dynamics of the microtubule [7]. Eribulin, in particular, works by chemically bonding the (+)-end of these microtubules, preventing the microtuble from expanding and thereby stopping it 'fishing' for a chromosome. Mitotic inhibitors such as eribulin are particularly effective at killing cancer cells rather than normal cells because cancer cells, by their nature, reproduce far more rapidly than normal cells, and are thus more susceptible to drugs which target cell division.
Despite Halavenís importance in treatment of various cancers, the drug has several unwanted side-effects. A decrease in white-blood-cell count, numbness, peripheral neuropathy, irregular heart-beat, and possible harm to unborn babies are some of the dangerous side-effects identified by the FDA.
![]()
![]()