![]()
Ethanol
Ancient intoxicant, spectacular solvent
and future fuel
![]()
Roderick Edmonds
Eton College, UK
![]()
Molecule of the Month December 2021
Also available: HTML version.
![]()

EthanolAncient intoxicant, spectacular solvent
|
 |
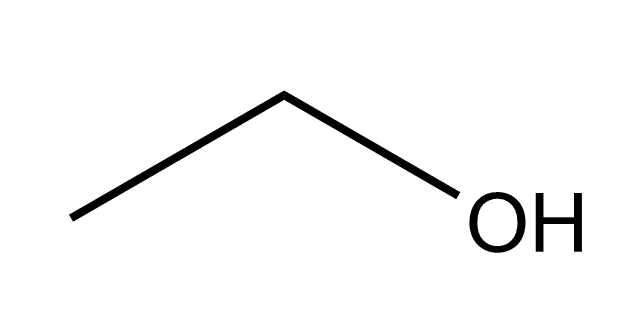 |
|
| Ethanol | Model of ethanol |
 Ethanol has been known about for a long time, hasn't it?
Ethanol has been known about for a long time, hasn't it?Yes. Long before William Hogarth’s 1751 drawing of Gin Lane (right), depicting the evils of alcohol abuse, Egyptian labourers were glad to slake their thirst with a pot of boozah, and micro-organisms were making the stuff millions of years before humans arrived. As you know, ethanol is the active ingredient in alcoholic drinks, such as wine, beer and spirits. Ethanol is a depressant for the central nervous system, and is actually classed as a psychoactive drug! Despite this, it is readily available and legal for sale in most countries, although usually with age restrictions.
When you drink ethanol, your body doesn't digest it immediately. Instead, it passes quickly into your bloodstream and travels to every part of your body. Ethanol affects your brain first, then your kidneys, lungs and liver, with the effects varying depending on your age, gender, weight and the type of drink (beer, whisky, etc). Ethanol slows the parts of your brain that control how your body works, and so affects your actions, your ability to make decisions, and your ability to stay in control. It also influences your mood and can make you feel depressed or aggressive.
As you drink more, and the ethanol concentration in your bloodstream increases, your body functions and behaviour can change. At first, you may feel happy and less inhibited (party time!), but after more drinks you'll probably start to slur your words, have blurred vision, lose your coordination, and possibly vomit (your body realises it has been 'poisoned 'and wants to get rid of the toxin fast!).
Unfortunately, there is no immediate way to sober up. It takes a few hours for your body to metabolise ethanol and clear it from your system in urine.
Drinking a lot more ethanol than your body can process can lead to acute alcohol poisoning. As well as many of the signs of drunkenness mentioned above, you may have more serious symptoms. These relate to ethanol being a depressant, i.e. it slows or stops some bodily functions. You may pass out, have a seizure, have trouble breathing or even stop breathing entirely, which means you'll die quickly unless treated! You may also exhibit a slow heart rate, clammy skin, and have dulled responses, such as no gag-reflex (which prevents choking). There are many cases of famous people who have died of the effects of alcohol poisoning, such as heart attacks, brain haemorrhages, or by choking on their own vomit because they were unconscious with no gag reflex. These include the AC/DC vocalist Bon Scott in 1980, Amy Winehouse in 2011 and the Led Zeppelin drummer John Bonham.
 |
 |
 |
| Bon Scott | Amy Winehouse [Photo: By Rama CC BY-SA 2.0 fr via Wikimedia commons] |
John Bonham |
And the problems with ethanol are compounded by its addictive properties. Heavy drinkers can become dependent upon the alcohol intake, which leads to chronic health issues such as kidney failure, liver cirrhosis, hepatitis, and death. Indeed, alcoholism reduces a person's life expectancy by ~10 years, and has become a serious problem all over the world. In 2016, the World Health Organization estimated that worldwide there were 380 million people with alcoholism, which equates to 5.1% of the population over 15 years of age. A total of 3.3 million deaths (~6% of all deaths!) are believed to be due to alcohol.
Yes. As the urgency of achieving global carbon neutrality becomes increasingly apparent, more ethanol is being burnt in engines – but as with all the possible alternatives to fossil fuels, there are still problems to overcome.
The key is fermentation, just as in the production of wine or beer. In fact, surplus wine in Europe is sometimes converted to bioethanol. Fermentation involves a series of enzyme-catalysed reactions which yeasts use to produce energy from glucose. The overall process is:
C6H12O6 → 2C2H5OH + 2CO2
It is one of the many examples of anaerobic respiration found among micro-organisms. It produces much less energy than aerobic respiration, where the glucose reacts with oxygen to give carbon dioxide and water, but yeast cells don’t need a lot of energy, and fermentation enables them to live where oxygen is not present.
The raw material for fermentation can be any sort of plant material which contains either sugars, or starch which can be broken down to sugars using enzymes. Those most widely used for bioethanol production are sugar-cane, sugar-beet, and maize (used mainly in the USA where it is known as “corn”). Although fermentation does produce CO2 as a by-product, this just regenerates some of the CO2 which the plants absorbed by photosynthesis – and you could trap it for storage or use in fizzy drinks rather than venting it to the atmosphere.
 UK Government information poster on ethanol in fuel. [Image: UK government Open License] |
You may have noticed that since October 2021, petrol in Britain is now labelled as “E10”, meaning that it contains 10% bioethanol. A normal petrol engine works just as well on this mixture. A car running on pure ethanol would need a modified engine, but adding ethanol to petrol is a step in the right direction. |
That all sounds good. What are the problems?Fermentation produces an aqueous solution containing up to about 15% ethanol, which must be purified by fractional distillation. This uses a considerable amount of energy. The crops will probably need fertiliser. Making and using fertilisers consumes significant amounts of fossil fuel and is responsible for about 2-3% of global warming due to greenhouse-gas emissions. The objection most often raised is that if crops are fermented to make ethanol, they are not available as food. Current research is directed at producing bioethanol from bagasse. |
 [Photo: Murgatroyd49, CC BY-SA 4.0 via Wikimedia Commons] |
 Waste straw, or source of biofuel? |
Bagasse? That doesn’t sound like a compliment!It usually means the waste material left after the sugar has been extracted from sugar-cane, but it can also apply to other inedible plant matter, such as straw or woodchips. These consist mainly of cellulose and other polymeric materials which yeast cannot metabolise. The idea is to develop efficient catalytic processes for breaking down these macromolecules into simple sugars. |
 We use lots of ethanol in the lab, don’t we?
We use lots of ethanol in the lab, don’t we?It’s very useful stuff. You may have made an ester such as ethyl ethanoate (see MOTM March 2003), or oxidised ethanol to ethanoic acid (see MOTM April 2016).
 In other experiments, ethanol is used as a solvent. It can bring together in solution many substances which would not normally mix. The OH group of ethanol can form hydrogen bonds, to water for example, while the C2H5 end is attracted to other sorts of molecules by van der Waals forces. A common sixth-form experiment is the reaction of a halogenoalkane such as 1-chlorobutane with the hydroxide ions in aqueous sodium hydroxide:
In other experiments, ethanol is used as a solvent. It can bring together in solution many substances which would not normally mix. The OH group of ethanol can form hydrogen bonds, to water for example, while the C2H5 end is attracted to other sorts of molecules by van der Waals forces. A common sixth-form experiment is the reaction of a halogenoalkane such as 1-chlorobutane with the hydroxide ions in aqueous sodium hydroxide:
C4H9Cl + OH- 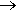 C4H9OH + Cl-
C4H9OH + Cl-
The snag is that sodium hydroxide will dissolve in water, but not in chlorobutane, and chlorobutane won’t mix with water. If ethanol is added, the two liquid layers merge together and the reaction becomes much easier (see photo, right).
Ethanol is included in many mouthwashes (see picture, left), because the antibacterials and other ingredients used would not dissolve if the only solvent present was water. (Don’t try drinking a bottle of mouthwash – it would be unpleasant and harmful).
Indicators such as methyl orange, phenolphthalein and universal indicator are usually dissolved in ethanol.
It almost certainly isn’t. Ethanol for industrial and lab use is often made by the addition reaction of ethene with steam, rather than by fermentation. As the reaction proceeds, some molecules of ethene will react with the ethanol produced earlier, rather than with steam. This gives ethoxyethane, C2H5OC2H5 (also known as the anaesthetic, ether) as an impurity. The ethoxyethane can be removed by distillation, but traces may remain, so the ethanol in drinks is normally made by fermentation.
Whichever way the ethanol is made, fractional distillation is used to separate it from the water present. Surprisingly, even the most careful fractional distillation will not produce pure ethanol from an aqueous mixture. The distillate is an azeotrope containing 96% ethanol and 4% water, which has a lower boiling point than either water or ethanol. Removing that last bit of water is not easy, so lab ethanol will probably be based on the azeotrope. And then methanol is deliberately added (about 5%). The resulting mixture is known as Industrial Denatured Alcohol. The toxic methanol is intended to make the mixture undrinkable, so that it can legally be sold without incurring the tax levied on ethanol for human consumption. “Methylated spirit” is a similar mixture with further additives including a purple dye, which is used for paint stripping or as a fuel for camping stoves (see photo, right). |
 Methylated spirit camping stove. |
There are several ways to measure the concentration of ethanol vapour in air or exhaled breath.
The original breathalysers, introduced in 1967, were small glass tubes containing orange crystals of potassium dichromate(VI), together with sulfuric acid. This mixture will oxidise ethanol, and the colour changes to green. You may have done this reaction in the lab (see MOTM April 2016). The breathalyser also includes a catalyst of silver nitrate. The picture below shows a home-made version which we are testing by bubbling the incoming air through some ethanol.

The next type of breathalyser involved measuring the infrared spectrum of ethanol. The spectrum below (red line) was recorded by a lab infrared spectrometer. The breathalyser is a simpler instrument which measures the infrared absorption at a number of specific frequencies, typically including those shown by the purple arrows. The broad peak on the left of the spectrum is not sampled because it is due to vibration of the O-H bond, and so will be masked by the water vapour present in exhaled breath.
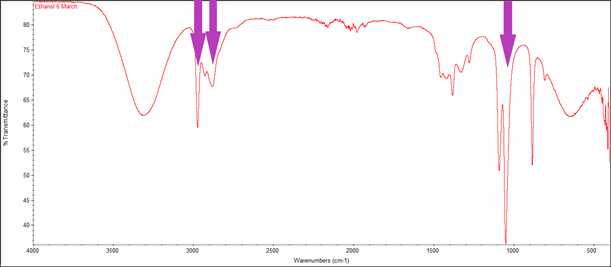
Infrared spectrum of ethanol showing some of the frequencies used by a breathalyser.
Infrared spectroscopy is a powerful analytical technique and its use is widespread; by linking it to telescopes we have detected ethanol molecules in space. The modern breathalyser is a type of “fuel cell”. The ethanol “fuel” reacts with oxygen to produce an electric current, rather than just heat. As with any electrical cell or battery, the overall redox reaction has to take place at two separate electrodes, with electrons flowing from one to the other. Oxidation of ethanol: C2H5OH + H2O Reduction of oxygen: O2 + 4H+ + 4e- |
 IR breathalyser technology used to detect ethanol in space? |
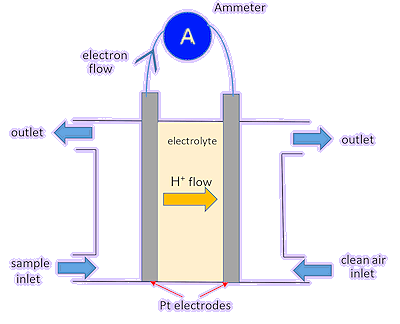 Right is a simple diagram. The electrodes are made of platinum, which catalyses the reactions as well as being an electrical conductor. The “electrolyte” allows H+ ions to flow across the cell at the same time as electrons flow through the external circuit. It’s the equivalent of the “salt bridge” which you will have seen if you have made an electrochemical cell in the lab.
Right is a simple diagram. The electrodes are made of platinum, which catalyses the reactions as well as being an electrical conductor. The “electrolyte” allows H+ ions to flow across the cell at the same time as electrons flow through the external circuit. It’s the equivalent of the “salt bridge” which you will have seen if you have made an electrochemical cell in the lab.
If you have been poisoned by ethylene glycol (ethane-1,2-diol), ethanol may be used as an antidote. Sometimes it works; sometimes it doesn’t. This really would be one of the silliest ways of trying to fix yourself a free drink. More detail at MOTM June 2018.
Ethanol is also commonly found as the active ingredient of antiseptic wipes and hand gels.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.17032703]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.17032703]