![]()
'Fensic'
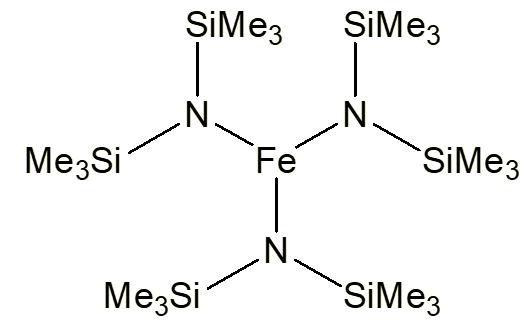
Tris[bis(trimethylsilyl)amido]iron(III)
or [Fe(N(SiMe3)2)3]
A pioneer of three-coordination
![]()
Simon Cotton
University of Birmingham
![]()
Molecule of the Month September 2025
Also available: HTML version.
![]()
|
'Fensic'Tris[bis(trimethylsilyl)amido]iron(III)
|
 |
It’s got three-coordinate iron.
An atom bound to three other atoms is found often with non-metals, like the BF3 you mention, or ammonia, NH3 (MOTM June 2013). The ‘coinage metals’ like Cu, Ag and Au in the (+1) oxidation state, and the succeeding Group IIB metals such as Cd and Hg in the (+2) state, have a few examples of such low coordination numbers.
Examples include [Au(CN)2]- (a species used in gold extraction), [AuCl(PPh3)2], Hg(CH3)2 (MOTM October 2003) and [HgI3]-.
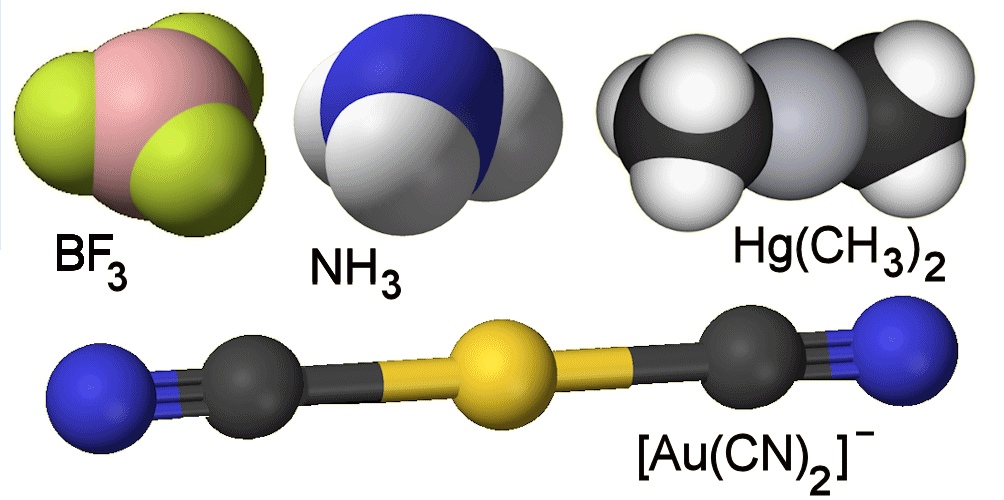
Various species with low coordination numbers.
The metals I mentioned have the d10 electron configuration, where such species with two- and three-coordination are known.
But such behaviour was unknown among the typical transition metals until half a century ago. Six coordination, and to a lesser extent four and five coordination, were the norm among compounds of these metals.
So what changed?Professor Don Bradley (1924-2014), of Queen Mary College, London, had made a speciality of studying transition metal alkoxides, [M(OR)n] since the days of his PhD; this remained an area of active interest throughout his research career. In the 1960s he extended this to the study of transition metal alkylamides, initially of the early transition metals like tungsten. In 1963, two German chemists named Bürger and Wannagat reported the synthesis of the very dark-green, almost black, air and moisture-sensitive [Fe(N(SiMe3)2)3]. Some years later, Don’s research group undertook the study of this compound and also made compounds of the bis(trimethyl)silylamide ligand with other 3d metals, also obtaining [M(N(SiMe3)2)3] (M = Sc, Ti, V, Cr); the Mn and Co compounds, completing the series for metals with d0-d6 electron configurations, were made by others some years later. What did they find?Crystal structures of these compounds, first obtained for [Fe(N(SiMe3)2)3] and reported in 1969, showed that the metal was bound to three nitrogen atoms, in a trigonal planar geometry. Evidently the very bulky SiMe3 groups were occupying so much space round the iron atom that no other ligands could approach. |
 Professor Don Bradley |
The synthesis employs the reaction between FeCl3 and LiN(SiMe3)2, in a ‘salt-elimination reaction’ (in THF or benzene-ether), as you might expect. The volatile product of the reaction can be purified by sublimation in vacuo at around 100°C; its volatility is in accord with its simple molecular structure.
But things are not as simple as the expected equation (below) would suggest:
FeCl3 + 3 LiN(SiMe3)2  [Fe(N(SiMe3)2)3] + 3 LiCl
[Fe(N(SiMe3)2)3] + 3 LiCl
The reaction by-product, expected to be just LiCl, contains iron(II) impurities. The most recent study of the synthesis has identified Li2FeCl4 and Li[Fe{N(SiMe3)2}3] among the by-products. On reflection, this is not too surprising, as FeCl3 is an oxidant, whilst LiN(SiMe3)2 is a reductant.
The electronic and magnetic properties are interesting. The magnetic moment is 5.91 μB, as expected for high-spin iron(III). The EPR spectrum shows that it is a high-spin iron(III) compound with a strong trigonal distortion, as the structure indicates. It is also a single-molecule magnet (SMM) at low temperatures. But I think of this compound as 'Fensic'.
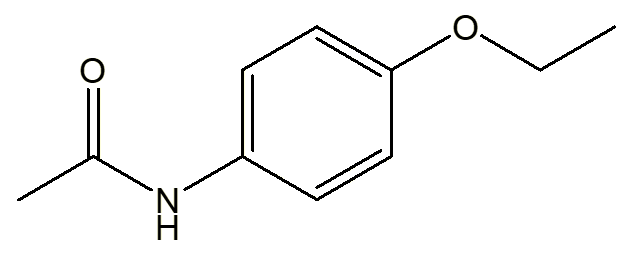
Well, the crystallographers at Queen Mary College, London, who first determined the structure of the compound, used six-letter labels to identify the compounds that they were studying. They called it 'Fensic'. This reflected the atomic linkage Fe-N-Si-C, but was also a play on the name of Phensic, a popular painkilling medicine, which was used by James Bond in Ian Fleming’s Moonraker and Thunderball.
For many years it used phenacetin as the active ingredient, which was synthesised about the same time as paracetamol (MOTM September 2020).
 |
 |
|
| Phenacetin | Phensic painiller advert |
Phenacetin probably owed its action to its decomposition in the body to paracetamol, so that it acted as a prodrug. However, it could also lead to the formation of the carcinogen p-phenetidine, and around 1970 this formulation of Phensic was withdrawn from use.
It has also been suggested that phenacetin could have been responsible for the premature death of Howard Hughes, the film magnate (and flying-boat designer).
 And is it anything to do with the ska/reggae group The Phensic?
And is it anything to do with the ska/reggae group The Phensic?No, but that is not all that should be said about the significance of [Fe(N(SiMe3)2)3].
The discovery that the bulky –N(SiMe3)2 ligand could congest the environment around a metal so much that three coordination was possible, led Don Bradley to look into the possibilities with other metals. Within two years, Joginder Singh Ghotra, a talented postdoctoral worker, had synthesised [Ln(N(SiMe3)2)3] for yttrium and all the lanthanides except for the short-lived promethium.
The slightly larger lanthanide ions in these compounds can form a four-coordinate phosphine-oxide adduct [Ln(N(SiMe3)2)3.Ph3PO], something that the 3d metals cannot do in their silylamides.
The compounds [Ln(N(SiMe3)2)3] differ in that in the solid state (but not in solution) they have a slightly pyramidal geometry at the lanthanide (though still being three coordinate). Calculations indicate that it is β-Si-C agostic interactions with the lanthanide that is responsible for this small pyramidal distortion, though others think that it is down to steric congestion. But that is another story.
![[Ln(N(SiMe3)2)3] [Ln(N(SiMe3)2)3]](Ln.gif)
[Ln(N(SiMe3)2)3], where Ln is a lanthanide metal.
Richard Andersen was later to extend three-coordination to [U(N(SiMe3)2)3]. So this iron compound links with the burgeoning research in low-coordination in lanthanide and actinide chemistry.
In memory of Don Bradley FRS and of Dick Copperthwaite.
![]()
![]()
![]() Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.27188625]
Back to Molecule of the Month page. [DOI:10.6084/m9.figshare.27188625]